Tick-borne encephalitis pathophysiology
|
Tick-borne encephalitis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tick-borne encephalitis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Tick-borne encephalitis pathophysiology |
|
Risk calculators and risk factors for Tick-borne encephalitis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Ilan Dock, B.S.
Overview
Tick-borne diseases are most often transmitted during a blood meal, either by a nymph or adult tick. Blood meals will occur with a higher rate of incidence from the late spring into the early fall, with the highest rate of tick-borne encephalitis viral (TBEV) infections during the early and late summer. The primary disease vector for TBEV is the Ixodidae tick family, found throughout most of Eurasia. The virus itself is a member of the flavivirus genus, with three distinct subtypes; Siberian, European, and Far Eastern. Pathogenesis occurs as the virus binds to a host cell receptor. Through a series of reactions, the virus enters the cell, is translated, and hi-jacks the host cell's replication machinery. After which immature virions are released within the cell, to ultimately spread infection. Viral replication will often occur within subcutaneous tissue. Replication also occurs within the lymph nodes, causing immense damage to the immune system. A later phase of the virus results in an infection of the CNS as the immune response increases the permeability of the blood-brain barrier.[1]
Life Cycle and Spread of Tick-Borne Diseases
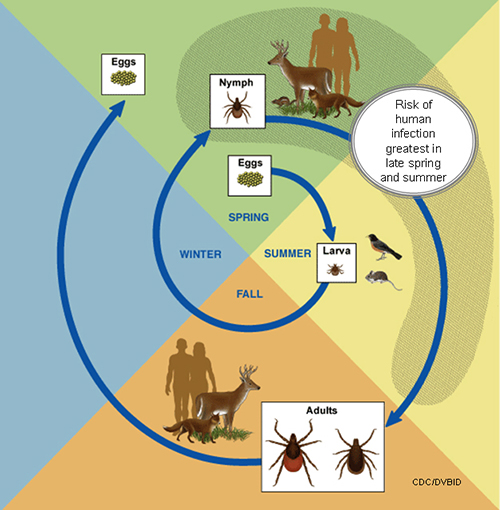
General Tick Life Cycle[2]
- A tick's life cycle is composed of four stages: hatching (egg), nymph (six legged), nymph (eight legged), and an adult.
- Ticks require blood meal to survive through their life cycle.
- Hosts for tick blood meals include mammals, birds, reptiles, and amphibians. Ticks will most likely transfer between different hosts during the different stages of their life cycle.
- Humans are most often targeted during the nymph and adult stages of the life cycle.
- Life cycle is also dependent on seasonal variation.
- Ticks will go from eggs to larva during the summer months, infecting bird or rodent host during the larval stage.
- Larva will infect the host from the summer until the following spring, at which point they will progress into the nymph stage.
- During the nymph stage, a tick will most likely seek a mammal host (including humans).
- A nymph will remain with the selected host until the following fall at which point it will progress into an adult.
- As an adult, a tick will feed on a mammalian host. However unlike previous stages, ticks will prefer larger mammals over rodents.
- The average tick life cycle requires three years for completion.
- Different species will undergo certain variations within their individual life cycles.
Spread of Tick-borne Diseases
- Ticks require blood meals in order to progress through their life cycles.
- The average tick requires 10 minutes to 2 hours when preparing a blood meal.
- Once feeding, releases anesthetic properties into its host, via its saliva.
- A feeding tube enters the host followed by an adhesive-like substance, attaching the tick to the host during the blood meal.
- A tick will feed for several days, feeding on the host blood and ingesting the host's pathogens.
- Once feeding is completed, the tick will seek a new host and transfer any pathogens during the next feeding process.[2]
Transmission
- The Ixodidae family of hard ticks have been reported as the vector and reservoir of the Tick-borne encephalitis virus.
- Other modes of transmission include the consumption of raw milk as well as vertical transmission from mother to fetus.[3]
Virology
- Member of the Falvivirus genus
- Flaviviridae family
- Three subtypes: Far East, European, and Siberian
- Viral strains are mostly homogeneous within infected European tick populations.
- Diversity exists within viral strains carried by Siberian and Far Eastern tick populations. Thus these populations host antigenic variations and a variety of subtypes.
- However the antigenic similarity within these populations allows for a generalized protection method among the different subtypes.[1]
Genomics
- (+)ssRNA genome enclosed in a capsid protein.
- Genome is protected by a lipid bilayer, provided by the host or target cell.
- Virus's physical attributes include a spherical particle with an approximate diameter of 50-60nm.
- The genome lacks a 3'-poly(A) tail, yet provides a 5' cap.
- In terms of length, the genome spans an average of 11kb.[1]
Pathogenesis
- The process begins as the virus binds to a host cell receptor.
- A host cell will internalize the virus using endocytosis.
- Post-endocytosis, acidification of the viral envelope causes conformation changes of the E protein, resulting in the attachment of the viral envelope to a endosomal vesicle.
- Once properly mounted on the endosomal vesicle, the viral envelope will release the viral nucleocapsid into the surrounding cytoplasm.
- Translation of the virus yields a 3414 amino acid long polyprotein.
- The polyprotein is cleaved by both cellular and viral proteases.
- The cleaving process results in three structural proteins called C, prM, and E as well as seven non-structural proteins.[1]
- The C protein forms a virion nucleocapsid through binding to viral DNA.
- The E protein is necessary as a ligand to cell receptors and as a fusion protein.
- The other non-structural proteins serve as proteases, polymerases, complement binding antigens, or function within the replication process.
- Finally the processes concludes as the positive-stranded genome is translated while the negative-strand of RNA provides grounds for the RNA replication process.
- Assembly of the virus occurs within the endoplasmic reticulum.
- Post-assembly immature virions are released within the cell.[1]
Viral pathway within a mammalian host
- Virus replication commonly occurs within subcutaneous tissue.
- Dendritic cells transport the virus to the lymph nodes.
- The virus replicates at a high rate within the lymph nodes, further travelling into the bloodstream.
- Lymphocytes suffer great reductions due to infection with the regional lymph nodes.
- Further infection of external tissues occur within the viremic phase
- The later phase results in the infection of the CNS.
- Furthermore a host's immune system will add to the severity of the infection, as resulting immune response includes inflammation CD8+ T-cells infiltrating the brain.
- Other immune responses such as the upregulation of proinflammatory cytokines increase the permeability of the blood-brain barrier.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Tick-borne Encephalitis Virus: A General Overview. http://cdn.intechopen.com/pdfs-wm/20866.pdf. Accessed February 4, 2016.
- ↑ 2.0 2.1 Life Cycle of Ticks that Bite Humans (2015). http://www.cdc.gov/ticks/life_cycle_and_hosts.html Accessed on December 30, 2015
- ↑ Tick-borne encephalitis transmission. http://www.cdc.gov/vhf/tbe/transmission/index.html Accessed February 5, 2016.