Tecovirimat
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tecovirimat is an inhibitor of the orthopoxvirus VP37 envelope wrapping protein that is FDA approved for the treatment of human smallpox disease in adults and pediatric patients weighing at least 13 kg. Common adverse reactions include headache, nausea, abdominal pain, and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Tecovirimat is indicated for the treatment of human smallpox disease caused by variola virus in adults and pediatric patients weighing at least 13 kg.
Limitations of Use
- The effectiveness of tecovirimat for treatment of smallpox disease has not been determined in humans because adequate and well-controlled field trials have not been feasible, and inducing smallpox disease in humans to study the drug’s efficacy is not ethical.
- Tecovirimat efficacy may be reduced in immunocompromised patients based on studies demonstrating reduced efficacy in immunocompromised animal models.
Dosage
- The recommended dosage of tecovirimat in adults and pediatric patients weighing at least 40 kg is 600 mg (three 200 mg capsules) taken twice daily orally for 14 days. Tecovirimat should be taken within 30 minutes after a full meal of moderate or high fat.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding tecovirimat Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding tecovirimat Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indication
- Tecovirimat is indicated for the treatment of human smallpox disease caused by variola virus in adults and pediatric patients weighing at least 13 kg.
Limitations of Use
- The effectiveness of tecovirimat for treatment of smallpox disease has not been determined in humans because adequate and well-controlled field trials have not been feasible, and inducing smallpox disease in humans to study the drug’s efficacy is not ethical.
- Tecovirimat efficacy may be reduced in immunocompromised patients based on studies demonstrating reduced efficacy in immunocompromised animal models.
Dosage
- The recommended dosage of tecovirimat in adults and pediatric patients weighing at least 40 kg is 600 mg (three 200 mg capsules) taken twice daily orally for 14 days. Tecovirimat should be taken within 30 minutes after a full meal of moderate or high fat.
- The recommended dosage for pediatric patients is based on weight starting at 13 kg as shown in TABLE 1 (see [Administration & Monitoring]). The dose should be given twice daily orally for 14 days and should be taken within 30 minutes after a full meal of moderate or high fat.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding tecovirimat Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding tecovirimat Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
Hypoglycemia When Co-Administered with Repaglinide
- Co-administration of repaglinide and tecovirimat may cause mild to moderate hypoglycemia. Monitor blood glucose and monitor for hypoglycemic symptoms when administering tecovirimat with repaglinide.
- In a drug interaction study, 10 of 30 healthy subjects experienced mild (6 subjects) or moderate (4 subjects) hypoglycemia following co-administration of repaglinide (2 mg) and tecovirimat. Symptoms resolved in all subjects after intake of food and/or oral glucose.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of tecovirimat has not been studied in patients with smallpox disease.
- The safety of tecovirimat was evaluated in 359 healthy adult subjects ages 18-79 years in a Phase 3 clinical trial. Of the subjects who received at least one 600 mg dose of tecovirimat, 59% were female, 69% were White, 28% were Black/African American, 1% were Asian, and 12% were Hispanic or Latino. Ten percent of the subjects who participated in the study were age 65 or older. Of these 359 subjects, 336 subjects received at least 23 of 28 doses of 600 mg tecovirimat in a twice daily regimen for 14 days.
Most Frequently Reported Adverse Reactions
- The most frequently reported adverse reactions were headache and nausea. Adverse reactions that occurred in at least 2% of subjects in the tecovirimat treatment group are shown in TABLE 2.

Adverse Reactions Leading to Discontinuation of Tecovirimat
- Six subjects (2%) had their treatment with tecovirimat discontinued due to adverse reactions. Each of these subject’s adverse reactions (with severity) is listed below:
Less Common Adverse Reactions
- Clinically significant adverse reactions that were reported in < 2% of subjects exposed to tecovirimat and at rates higher than subjects who received placebo are listed below:
- Gastrointestinal: dry mouth, chapped lips, dyspepsia, eructation, oral paresthesia
- General and administration site: pyrexia, pain, chills, malaise, thirst
- Investigations: abnormal electroencephalogram, hematocrit decreased, hemoglobin decreased, heart rate increased
- Musculoskeletal and connective tissue: arthralgia, osteoarthritis
- Nervous system: migraine, disturbance in attention, dysgeusia, paresthesia
- Psychiatric: depression, dysphoria, irritability, panic attack
- Respiratory, Thoracic and Mediastinal Disorders: oropharyngeal pain
- Skin and subcutaneous tissue: palpable purpura, rash, pruritic rash, facial redness, facial swelling, pruritus
Postmarketing Experience
There is limited information regarding Tecovirimat Postmarketing Experience in the drug label.
Drug Interactions
Effect of Tecovirimat on Other Drugs
- Tecovirimat is a weak inducer of cytochrome P450 (CYP)3A and a weak inhibitor of CYP2C8 and CYP2C19. However, the effects are not expected to be clinically relevant for most substrates of those enzymes based on the magnitude of interactions and the duration of treatment of tecovirimat. See TABLE 3 for clinical recommendations for select sensitive substrates.
Established Drug Interactions
- TABLE 3 provides a listing of established or significant drug interactions.

Drugs Without Clinically Significant Interactions With Tecovirimat
- Based on a drug interaction study, no clinically significant drug interactions have been observed when tecovirimat is co-administered with bupropion, flurbiprofen, or omeprazole.
Vaccine Interactions
- No vaccine-drug interaction studies have been performed in human subjects. Some animal studies have indicated that co-administration of tecovirimat at the same time as live smallpox vaccine (vaccinia virus) may reduce the immune response to the vaccine. The clinical impact of this interaction on vaccine efficacy is unknown.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- No adequate and well-controlled studies in pregnant women were conducted; therefore there are no human data to establish the presence or absence of tecovirimat associated risk.
- In animal reproduction studies, no embryofetal developmental toxicity was observed in mice during the period of organogenesis at tecovirimat exposures (area under the curve [AUC]) up to 23 times higher than human exposure at the recommended human dose (RHD). In rabbits, no embryofetal developmental toxicity was observed during organogenesis at tecovirimat exposures (AUC) less than human exposures at the RHD. In a mouse pre-/post-natal development study, no toxicities were observed at maternal tecovirimat exposures up to 24 times higher than human exposure at the RHD.
- The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Animal Data
- Tecovirimat was administered orally to pregnant mice at doses up to 1,000 mg/kg/day from gestation Days 6-15. No embryofetal toxicities were observed at doses up to 1,000 mg/kg/day (approximately 23 times higher than human exposure at the RHD).
- Tecovirimat was administered orally to pregnant rabbits at doses up to 100 mg/kg/day from gestation Days 6-19. No embryofetal toxicities were observed at doses up to 100 mg/kg/day (0.4 times the human exposure at the RHD).
- In the pre-/post-natal development study, tecovirimat was administered orally to pregnant mice at doses up to 1,000 mg/kg/day from gestation Day 6 to post-natal Day 20. No toxicities were observed at doses up to 1,000 mg/kg/day (approximately 24 times higher than human exposure at the RHD).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tecovirimat in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tecovirimat during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data to assess the effect on milk production, the presence of the drug in human milk, and/or the effects on the breastfed child. When administered to lactating mice, tecovirimat was present in the milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for tecovirimat and any potential adverse effects on the breastfed child from tecovirimat or from the underlying maternal condition.
Data
- In a lactation study at doses up to 1,000 mg/kg/day, mean tecovirimat milk to plasma ratios up to approximately 0.8 were observed at 6 and 24 hours post-dose when administered orally to mice on lactation Day 10 or 11.
Pediatric Use
- As in adults, the effectiveness of tecovirimat in pediatric patients is based solely on efficacy studies in animal models of orthopoxvirus disease. As exposure of healthy pediatric subjects to tecovirimat with no potential for direct clinical benefit is not ethical, pharmacokinetic simulation was used to derive dosing regimens that are predicted to provide pediatric patients with exposures comparable to the observed exposure in adults receiving 600 mg twice daily. The dosage for pediatric patients is based on weight.
Geriatic Use
- Clinical studies of tecovirimat did not include sufficient numbers of subjects aged 65 and over to determine whether the safety profile of tecovirimat is different in this population compared to younger subjects. Of the 359 subjects in the clinical study of tecovirimat, 10% (36/359) were ≥ 65 years of age, and 1% (4/359) were ≥ 75 years of age. No alteration of dosing is needed for patients ≥ 65 years of age.
Gender
There is no FDA guidance on the use of Tecovirimat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tecovirimat with respect to specific racial populations.
Renal Impairment
- No dosage adjustment is required for patients with mild, moderate or severe renal impairment or patients with end stage renal disease (ESRD) requiring hemodialysis
Hepatic Impairment
- No dosage adjustment is required for patients with mild, moderate or severe hepatic impairment (Child Pugh Class A, B, or C).
Females of Reproductive Potential and Males
Infertility
- There are no data on the effect of tecovirimat on female and male reproductive potential in humans. Decreased fertility due to testicular toxicity was observed in male mice.
Immunocompromised Patients
There is no FDA guidance one the use of Tecovirimat in patients who are immunocompromised.
Administration and Monitoring
Administration
Dosage for Adults and Pediatric Patients Weighing at Least 40 kg
- The recommended dosage of tecovirimat in adults and pediatric patients weighing at least 40 kg is 600 mg (three 200 mg capsules) taken twice daily orally for 14 days. Tecovirimat should be taken within 30 minutes after a full meal of moderate or high fat.
Dosage for Pediatric Patients
- The recommended dosage for pediatric patients is based on weight starting at 13 kg as shown in TABLE 1. The dose should be given twice daily orally for 14 days and should be taken within 30 minutes after a full meal of moderate or high fat.
Preparation for Administration to Pediatrics and Those Who Cannot Swallow Capsules
- Tecovirimat capsules can be administered by carefully opening the capsule and mixing the entire contents in 30 mL of liquid (e.g., milk, chocolate milk) or soft food (e.g., apple sauce, yogurt). The entire mixture should be administered within 30 minutes of its preparation.

Monitoring
There is limited information regarding Tecovirimat Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tecovirimat and IV administrations.
Overdosage
- There is no clinical experience with overdosage of tecovirimat. In case of overdosage, monitor patients for any signs or symptoms of adverse effects. Hemodialysis will not significantly remove tecovirimat in overdosed patients.
Pharmacology
| |
Tecovirimat
| |
| Systematic (IUPAC) name | |
| N-{3,5-Dioxo-4-azatetracyclo[5.3.2.0{2,6}.0{8,10}]dodec-11-en-4- yl}-4-(trifluoromethyl)benzamide | |
| Identifiers | |
| CAS number | ? |
| ATC code | J05 |
| PubChem | ? |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| SMILES | & |
| Synonyms | ST-246 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Prescription only (US)[1] |
| Routes | By mouth |
Mechanism of Action
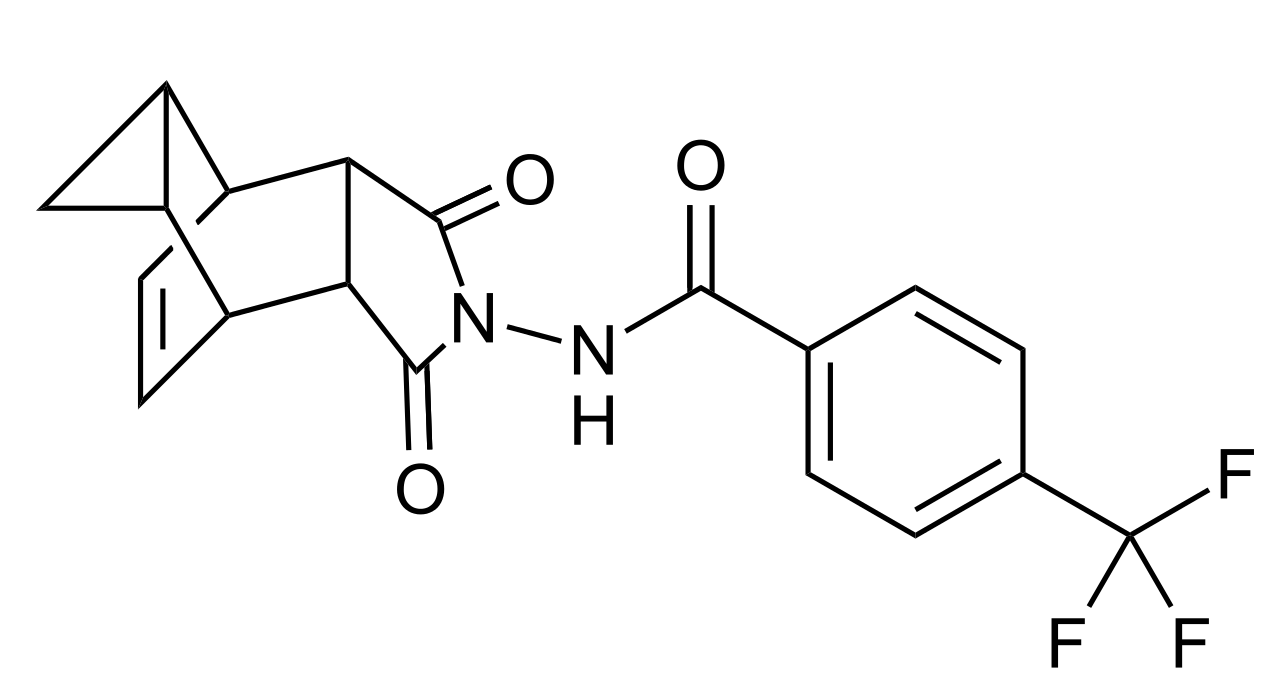
Structure
- The chemical formula is C19H15F3N2O3·H2O representing a molecular weight of 394.35 g/moL. The molecular structure is as follows:

Pharmacodynamics
Cardiac Electrophysiology
- Tecovirimat does not prolong the QT interval to any clinically relevant extent at the anticipated therapeutic exposure.
Pharmacokinetics
- At the approved recommended dosage, the mean steady-state values of tecovirimat AUC0-24hr, Cmax, and Cmin are 28791 hr·ng/mL (CV: 35%), 2106 ng/mL (CV: 33%), and 587 ng/mL (CV: 38%), respectively. Tecovirimat steady-state AUC is achieved by Day 6. Refer to TABLE 4 for pharmacokinetic parameters of tecovirimat.

Comparison of Animal and Human PK Data to Support Effective Human Dose Selection
- Because the effectiveness of tecovirimat cannot be tested in humans, a comparison of tecovirimat exposures achieved in healthy human subjects to those observed in animal models of orthopoxvirus infection (nonhuman primates and rabbits infected with monkeypox virus and rabbitpox virus, respectively) in therapeutic efficacy studies was necessary to support the dosage regimen of 600 mg twice daily for treatment of smallpox disease in humans. Humans achieve greater systemic exposure (AUC, Cmax, and Cmin) of tecovirimat following a twice daily dose of 600 mg when compared to the therapeutic exposures in these animal models.
Specific Populations
- No clinically significant differences in the pharmacokinetics of tecovirimat were observed based on age, sex, ethnicity, renal impairment (based on estimated GFR), or hepatic impairment (Child Pugh Scores A, B or C).
Pediatric Patients
- Tecovirimat pharmacokinetics has not been evaluated in pediatric patients. The recommended pediatric dosing regimen is expected to produce tecovirimat exposures that are comparable to those in adult subjects based on a population pharmacokinetic modeling and simulation approach.
Drug Interaction Studies
- The effect of tecovirimat on the exposure of co-administered drugs are shown in TABLE 5.

- No pharmacokinetic changes were observed for the following drug when co-administered with tecovirimat: flurbiprofen.
- Cytochrome P450 (CYP) Enzymes: Tecovirimat is a weak inhibitor of CYP2C8 and CYP2C19, and a weak inducer of CYP3A4. Tecovirimat is not an inhibitor or an inducer of CYP2B6 or CYP2C9.
In Vitro Studies Where Drug Interaction Potential Was Not Further Evaluated Clinically
- CYP Enzymes: Tecovirimat is not an inhibitor of CYP1A2, CYP2D6, CYP2E1 or CYP3A4, and is not an inducer of CYP1A2. Tecovirimat is not a substrate for CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A4.
- UGT Enzymes: Tecovirimat is a substrate of UGT1A1 and UGT1A4.
- Transporter Systems: Tecovirimat inhibited Breast Cancer Resistance Protein (BCRP) in vitro.
- Tecovirimat is not an inhibitor of P-glycoprotein (P-gp), organic anion transporting polypeptides 1B1 and 1B3 (OATP1B1 and OATP1B3), organic anion transporter 1 (OAT1), OAT3, and organic cation transporter 2 (OCT2). Tecovirimat is not a substrate for P-gp, BCRP, OATP1B1, and OATP1B3.
Microbiology
Mechanism of Action
- Tecovirimat targets and inhibits the activity of the orthopoxvirus VP37 protein (encoded by and highly conserved in all members of the orthopoxvirus genus) and blocks its interaction with cellular Rab9 GTPase and TIP47, which prevents the formation of egress-competent enveloped virions necessary for cell-to-cell and long-range dissemination of virus.
Activity in Cell Culture
- In cell culture assays, the effective concentrations of tecovirimat resulting in a 50% reduction in virus-induced cytopathic effect (EC50), were 0.016-0.067 µM, 0.014-0.039 µM, 0.015 µM, and 0.009 µM, for variola, monkeypox, rabbitpox, and vaccinia viruses, respectively. Ranges given for variola and monkeypox viruses are reflective of results from multiple strains assayed.
Resistance
- There are no known instances of naturally occurring tecovirimat resistant orthopoxviruses, although tecovirimat resistance may develop under drug selection. Tecovirimat has a relatively low resistance barrier, and certain amino acid substitutions in the target VP37 protein can confer large reductions in tecovirimat antiviral activity. The possibility of resistance to tecovirimat should be considered in patients who either fail to respond to therapy or who develop recrudescence of disease after an initial period of responsiveness.
- Cross Resistance: There are no other antiviral drugs approved for the treatment of variola (smallpox) virus infection.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
- Carcinogenicity studies have not been conducted with tecovirimat.
- Tecovirimat was not genotoxic in in vitro or in vivo assays, including a bacterial reverse mutation assay, a mammalian mutagenicity assay in mouse lymphoma L5178Y/TK± cells, and in an in vivo mouse micronucleus study.
Impairment of Fertility
- In a fertility and early embryonic development study in mice, no effects of tecovirimat on female fertility were observed at tecovirimat exposures (AUC) approximately 24 times higher than human exposure at the RHD. In male mice, decreased male fertility associated with testicular toxicity (increased percent abnormal sperm and decreased sperm motility) was observed at 1,000 mg/kg/day (approximately 24 times the human exposure at the RHD).
Animal Toxicology and/or Pharmacology
- In a repeat-dose toxicology study in dogs, convulsions (tonic and clonic) were observed in one animal within 6 hours of a single dose of 300 mg/kg (approximately 4 times higher than the highest observed human exposure at the RHD based on Cmax). Electroencephalography (EEG) findings in this animal were consistent with seizure activity during the observed convulsions. Tremors, which were considered non-adverse, were observed at 100 mg/kg/dose (similar to the highest observed human exposure at the RHD based on Cmax), although no convulsions or EEG findings were observed at this dose.
Clinical Studies
Overview
- The effectiveness of tecovirimat for treatment of smallpox disease has not been determined in humans because adequate and well-controlled field trials have not been feasible, and inducing smallpox disease in humans to study the drug’s efficacy is not ethical. Therefore, the effectiveness of tecovirimat for treatment of smallpox disease was established based on results of adequate and well-controlled animal efficacy studies of non-human primates and rabbits infected with non-variola orthopoxviruses. Survival rates observed in the animal studies may not be predictive of survival rates in clinical practice.
Study Design
- Efficacy studies were conducted in cynomolgus macaques infected with monkeypox virus, and New Zealand white (NZW) rabbits infected with rabbitpox virus. The primary efficacy endpoint for these studies was survival. In non-human primate studies, cynomolgus macaques were lethally challenged intravenously with 5 x 107 plaque-forming units of monkeypox virus; tecovirimat was administered orally once daily at a dose level of 10 mg/kg for 14 days, starting at Day 4, 5 or 6 post-challenge. In rabbit studies, NZW rabbits were lethally challenged intradermally with 1,000 plaque-forming units of rabbitpox virus; tecovirimat was administered orally once daily for 14 days at a dose level of 40 mg/kg, starting at Day 4 post-challenge. The timing of tecovirimat dosing in these studies was intended to assess efficacy when treatment is initiated after animals have developed clinical signs of disease, specifically dermal pox lesions in cynomolgus macaques, and fever in rabbits. Clinical signs of disease were evident in some animals at Day 2-3 post-challenge but were evident in all animals by Day 4 post-challenge. Survival was monitored for 3-6 times the mean time to death for untreated animals in each model.
Study Results
- Treatment with tecovirimat for 14 days resulted in statistically significant improvement in survival relative to placebo, except when given to cynomolgus macaques starting at Day 6 post-challenge (TABLE 6).

How Supplied
- Each tecovirimat capsule contains 200 mg of tecovirimat. Tecovirimat capsules are hard gelatin with an opaque orange body imprinted in white ink with “SIGA” followed by the SIGA logo followed by “®”, and an opaque black cap imprinted in white ink with "ST-246®", containing white to off-white powder. Each bottle contains 42 capsules (NDC 50072-200-42) with an induction seal and child-resistant cap.
Storage
- Store capsules in the original bottle at 20°C to 25°C (68°F to 77°F); excursions permitted 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Tecovirimat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Tecovirimat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
Efficacy Based on Animal Models Alone
- Inform patients that the efficacy of tecovirimat is based solely on efficacy studies demonstrating a survival benefit in animals and that the effectiveness of tecovirimat has not been tested in humans with smallpox disease.
Important Dosage and Administration Information
- Advise patients to take tecovirimat as directed within 30 minutes of eating a full meal of moderate or high fat. Inform patients to take tecovirimat for the entire duration without missing or skipping a dose.
Drug Interactions
- Inform patients that tecovirimat may interact with other drugs. Advise patients to report to their healthcare provider the use of other prescription drugs. Co-administration of tecovirimat with repaglinide may cause hypoglycemia.
Patient Package Insert

Precautions with Alcohol
Alcohol-Tecovirimat interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Tecovirimat Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.