Sulfacetamide (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sulfacetamide (ophthalmic) is a antibacterial agent that is FDA approved for the treatment of conjunctivitis and other superficial ocular infections due to susceptible microorganisms, and as an adjunctive in systemic sulfonamide therapy of trachoma. Common adverse reactions include corneal ulcers, application site irritation, stinging and burning.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- For the treatment of conjunctivitis and other superficial ocular infections due to susceptible microorganisms, and as an adjunctive in systemic sulfonamide therapy of trachoma:
- Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus (viridans group), Haemophilus influenzae, Klebsiella species, and Enterobacter species.
- Topically applied sulfonamides do not provide adequate coverage against Neisseria species, Serratia marcescens and Pseudomonas aeruginosa. A significant percentage of staphylococcal isolates are completely resistant to sulfa drugs.
Dosing Information
For conjunctivitis and other superficial ocular infections:
- Instill one or two drops into the conjunctival sac(s) of the affected eye(s) every two to three hours initially. Dosages may be tapered by increasing the time interval between doses as the condition responds. The usual duration of treatment is seven to ten days.
For Trachoma:
- Instill two drops into the conjunctival sac(s) of the affected eye(s) every two hours. Topical administration must be accompanied by systemic administration.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sulfacetamide (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sulfacetamide (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Sulfacetamide (ophthalmic) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sulfacetamide (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sulfacetamide (ophthalmic) in pediatric patients.
Contraindications
- Hypersensitivity to sulfonamides or to any ingredient of the preparation.
Warnings
- For topical eye use only - not for injection.
- Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including stevens-johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia and other blood dyscrasias. Sensitizations may recur when a sulfonamide is readministered, irrespective of the route of administration. Sensitivity reactions have been reported in individuals with no prior history of sulfonamide hypersensitivity. At the first sign of hypersensitivity, skin rash or other serious reaction, discontinue use of this preparation.
PRECAUTIONS:
General:
- Prolonged use of topical anti-bacterial agents may give rise to overgrowth of nonsusceptible organisms including fungi. Bacterial resistance to sulfonamides may also develop.
- The effectiveness of sulfonamides may be reduced by the para-aminobenzoic acid present in purulent exudates.
- Sensitization may recur when a sulfonamide is readministered irrespective of the route of administration, and crosssensitivity between different sulfonamides may occur.
- At the first sign of hypersensitivity, increase in purulent discharge, or aggravation of inflammation or pain, the patient should discontinue use of the medication and consult a physician.
Adverse Reactions
Clinical Trials Experience
- Bacterial and fungal corneal ulcers have developed during treatment with sulfonamide ophthalmic preparations.
- The most frequently reported reactions are local irritation, stinging and burning. Less commonly reported reactions include non-specific conjunctivitis, conjunctival hyperemia, secondary infections and allergic reactions.
- Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias.
Postmarketing Experience
There is limited information regarding Sulfacetamide (ophthalmic) Postmarketing Experience in the drug label.
Drug Interactions
- Sulfacetamide preparations are incompatible with silver preparations.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with sulfonamide ophthalmic preparations. Kernicterus may occur in the newborn as a result of treatment of a pregnant woman at term with orally administered sulfonamides. There are no adequate and well-controlled studies of sulfonamide ophthalmic preparations in pregnant women and it is not known whether topically applied sulfonamides can cause fetal harm when administered to a pregnant woman. This product should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sulfacetamide (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sulfacetamide (ophthalmic) during labor and delivery.
Nursing Mothers
- Systemically administered sulfonamides are capable of producing kernicterus in infants of lactating women. Because of the potential for the development of kernicterus in neonates, a decision should be made whether to discontinue nursing or discontinue the drug taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in children below the age of two months have not been established.
Geriatic Use
There is no FDA guidance on the use of Sulfacetamide (ophthalmic) in geriatric settings.
Gender
There is no FDA guidance on the use of Sulfacetamide (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sulfacetamide (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sulfacetamide (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sulfacetamide (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sulfacetamide (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sulfacetamide (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic
Monitoring
There is limited information regarding Sulfacetamide (ophthalmic) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sulfacetamide (ophthalmic) and IV administrations.
Overdosage
There is limited information regarding Sulfacetamide (ophthalmic) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| Template:Px | |
| |
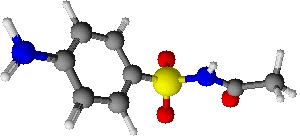
Sulfacetamide (ophthalmic)
| |
| Systematic (IUPAC) name | |
| N-[(4-aminophenyl)sulfonyl]acetamide | |
| Identifiers | |
| CAS number | |
| ATC code | D10 S01AB04 (WHO), Template:ATCvet |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 214.243 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 7 to 12.8 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Sulfacetamide Sodium Ophthalmic Solution USP, 10%, is a sterile, topical, anti-bacterial agent for ophthalmic use. The active ingredient is represented by the following structural formula:
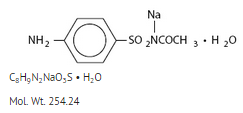
- Chemical name: N-Sulfanilylacetamide monosodium salt monohydrate.
Structure
- The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, based on sulfonamides acting as a competitive inhibitor of para-aminobenzoic acid (PABA) utilization, an essential component for bacterial growth.
Pharmacodynamics
Microbiology:
- The sulfonamides are bacteriostatic agents and the spectrum of activity is similar for all. Sulfonamides inhibit bacterial synthesis of dihydrofolic acid by preventing the condensation of the pteridine with aminobenzoic acid through competitive inhibition of the enzyme dihydropteroate synthetase. Resistant strains have altered dihydropteroate synthetase with reduced affinity for sulfonamides or produce increased quantities of aminobenzoic acid.
- Topically applied sulfonamides are considered active against susceptible strains of the following common bacterial eye pathogens: Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus (viridans group), Haemophilus influenzae, Klebsiella species, and Enterobacter species.
Topically applied sulfonamides do not provide adequate coverage against Neisseria species, Serratia marcescens and Pseudomonas aeruginosa. A significant percentage of staphylococcal isolates are completely resistant to sulfa drugs.
Pharmacokinetics
There is limited information regarding Sulfacetamide (ophthalmic) Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility:
- No studies have been conducted in animals or in humans to evaluate the possibility of these effects with ocularly administered sulfacetamide. Rats appear to be especially susceptible to the goitrogenic effects of sulfonamides, and long-term oral administration of sulfonamides has resulted in thyroid malignancies in these animals.
Clinical Studies
There is limited information regarding Sulfacetamide (ophthalmic) Clinical Studies in the drug label.
How Supplied
Sulfacetamide Sodium Ophthalmic Solution USP, 10%, is supplied in a plastic squeeze bottle with a controlled drop tip in the following size:
15 mL bottle - Prod. No. 03011
DO NOT USE IF IMPRINTED “Protective Seal” WITH YELLOW symbol IS NOT INTACT
Sulfonamide solutions, on long standing, will darken in color and should be discarded.
KEEP OUT OF REACH OF CHILDREN.
Revised November 2007
Bausch & Lomb Incorporated Tampa, FL 33637
©Bausch & Lomb Incorporated
9113200 (Folded) 9113300 (Flat)
Storage
- Store between 2° - 30°C (36° - 86°F).
Images
Drug Images
{{#ask: Page Name::Sulfacetamide (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
NDC 24208-670-04
Bausch & Lomb
Sulfacetamide
Sodium
Ophthalmic
Solution
USP, 10%
(Sterile)
Rx only
[icon- eye]
[icon- 10%]
[icon- solution]
[icon- 15 mL]

{{#ask: Label Page::Sulfacetamide (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- To avoid contamination, do not touch tip of container to eye, eyelid or any surface.
Precautions with Alcohol
Alcohol-Sulfacetamide (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Bleph-10
- Ocu-Sul
Look-Alike Drug Names
There is limited information regarding Sulfacetamide (ophthalmic) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.