Smallpox laboratory tests
|
Smallpox Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Smallpox laboratory tests On the Web |
|
American Roentgen Ray Society Images of Smallpox laboratory tests |
|
Risk calculators and risk factors for Smallpox laboratory tests |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Overview
The polymerase chain reaction test and the growth of the virus in specific cell cultures allow the identification of the smallpox virus.
Laboratory Findings
There are laboratory tests to specifically identify the smallpox virus. However, in a patient with low to medium level of suspicion of smallpox, a false-positive result would have great personal and social impacts. Therefore, these tests must be used cautiously, and according to certain guidelines.[1][2][3][4]
Laboratory Criteria for Confirmation
For laboratory testing, the following specimens may be requested:
For patients defined as high risk for smallpox, the following tests are available:[1]
Polymerase Chain Reaction (PCR)
- Identifies smallpox virus DNA in the clinical specimen
Electron Microscopy
- Identifies typical virions with brick shape
- Can not distinguish smallpox virus DNA from other orthopoxviruses
Serologic Tests
- Not able to distinguish smallpox virus from other orthopoxviruses
Cell Culture
- Smallpox virus may be grown in different cell lines, producing characteristic pocks on some cells of chicken embryos, enabling species identification
Laboratory diagnostic testing for smallpox virus should be conducted in a CDC Laboratory Response Network (LRN) laboratory utilizing LRN-approved PCR tests and protocols for this virus. Initial confirmation of a smallpox outbreak requires additional testing by the CDC.
Note: Generic orthopox in PCR and negative stain electron microscopy (EM) identification of a pox virus in a clinical specimen, are suggestive of anorthopox virus infection, but not diagnostic for smallpox.
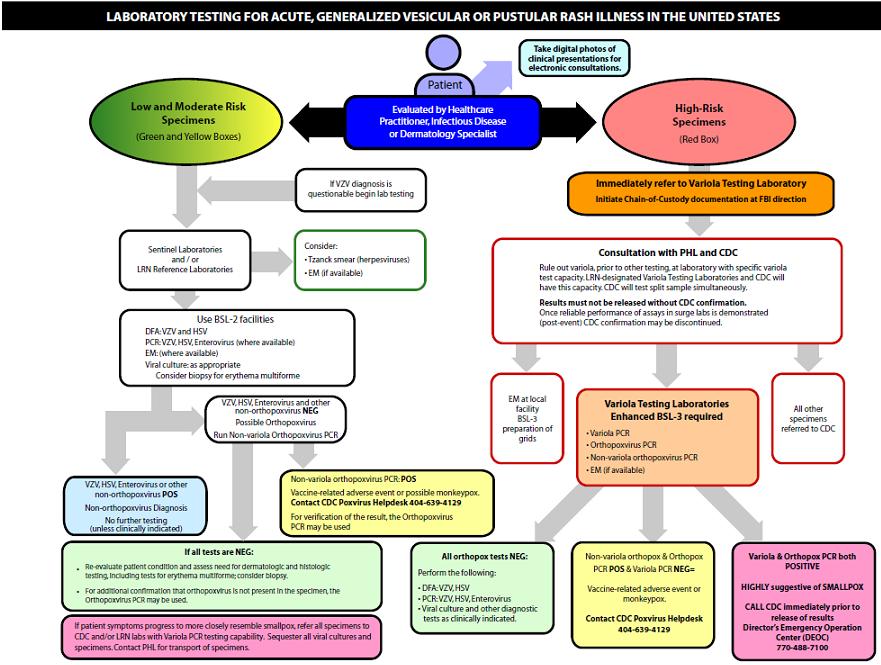
Laboratory Test Algorithm
The algorithm below describes the laboratory protocol for a patient who presents with a generalized vesicular or pustular rash. This algorithm is based on the risk level of the patient to be infected with smallpox virus.[1]
References
- ↑ 1.0 1.1 1.2 Moore, Zack S; Seward, Jane F; Lane, J Michael (2006). "Smallpox". The Lancet. 367 (9508): 425–435. doi:10.1016/S0140-6736(06)68143-9. ISSN 0140-6736.
- ↑ Seward JF, Galil K, Damon I, Norton SA, Rotz L, Schmid S; et al. (2004). "Development and experience with an algorithm to evaluate suspected smallpox cases in the United States, 2002-2004". Clin Infect Dis. 39 (10): 1477–83. doi:10.1086/425500. PMID 15546084.
- ↑ Besser JM, Crouch NA, Sullivan M (2003). "Laboratory diagnosis to differentiate smallpox, vaccinia, and other vesicular/pustular illnesses". J Lab Clin Med. 142 (4): 246–51. doi:10.1016/S0022-2143(03)00146-X. PMID 14625530.
- ↑ Madeley, CR (2003). "Diagnosing smallpox in possible bioterrorist attack". The Lancet. 361 (9352): 97–98. doi:10.1016/S0140-6736(03)12241-6. ISSN 0140-6736.
- ↑ "Public Health Image Library (PHIL), Centers for Disease Control and Prevention" (PDF).