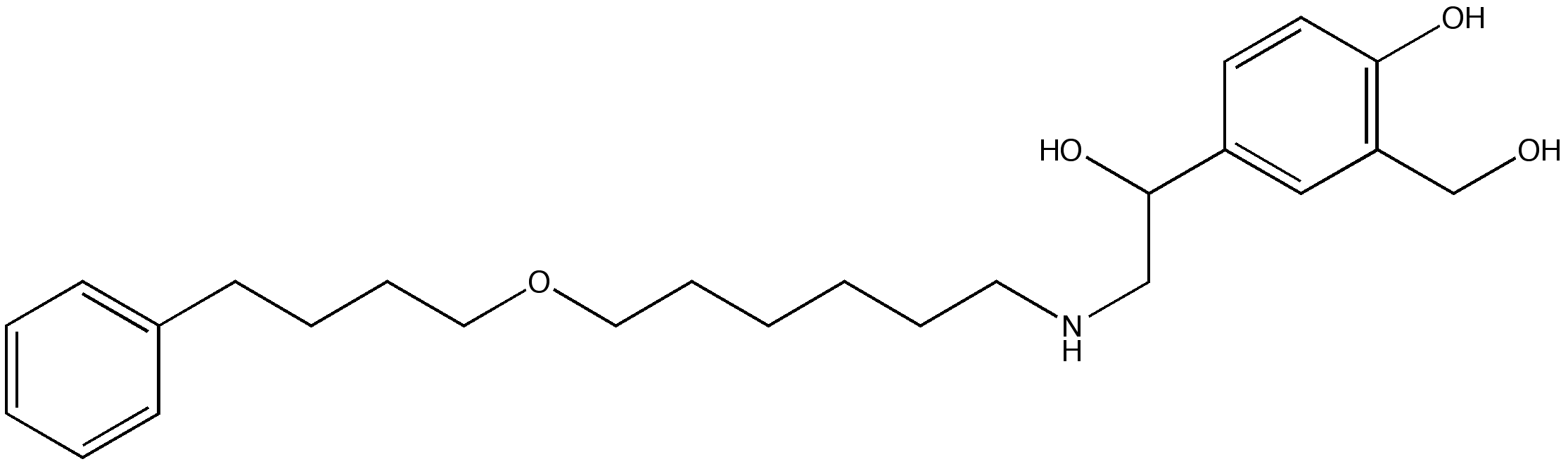
Salmeterol detailed information
 | |
| Clinical data | |
|---|---|
| Routes of administration | Inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 96% |
| Metabolism | hepatic CYP3A4 |
| Elimination half-life | 5.5 Hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C25H37NO4 |
| Molar mass | 415.57 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Salmeterol is a long-acting beta2-adrenergic receptor agonist drug that is currently prescribed for the treatment of asthma and chronic obstructive pulmonary disease COPD. It is currently available as a metered-dose inhaler (MDI) or a proprietary device inhaler.
Indications
It is a long acting beta-adrenoceptor agonist (LABA), usually only prescribed for severe persistent asthma following previous treatment with a short-acting beta agonist such as salbutamol and is prescribed concurrently with a corticosteroid, such as beclometasone. The primary noticeable difference of salmeterol to salbutamol is that the duration of action lasts approximately 12 hours in comparison with 4–6 hours of salbutamol.
When used regularly every day as prescribed, inhaled salmeterol decreases the number and severity of asthma attacks. However, like all LABA medications, it is not for use for relieving an asthma attack that has already started.
Inhaled salmeterol works like other beta 2-agonists, causing bronchodialation by relaxing the smooth muscle in the airway so as to treat the exacerbation of asthma. The long duration of formoterol action occurs by the formoterol molecules initially diffusing into the plasma membrane of the lung cells, and then slowly being released back outside the cell where they can come into contact with the beta-2 adrenoceptors. Formoterol has been demonstrated to have a faster onset of action than salmeterol as a result of a lower lipophilicity, and has also been demonstrated to be more potent—a 12 µg dose of formoterol has been demonstrated to be equivalent to a 50 µg dose of salmeterol.
Formulations
Currently available long-acting beta2-adrenoceptor agonists include salmeterol, formoterol, bambuterol, and sustained-release oral albuterol. Combinations of inhaled steroids and long-acting bronchodilators are becoming more widespread; the most common combination currently in use is fluticasone/salmeterol (Advair in the United States, Seretide in the UK).
History and concerns

Salmeterol, marketed and manufactured by GlaxoSmithKline, in the 1980s and was released as Serevent in 1990. However, the product is under license from Allen & Hanburys.(UK)
In November of 2005, the American FDA released a health advisory[2], alerting the public to findings that show the use of Long-acting β2-agonists could lead to a worsening of symptoms, and in some cases death.
Whilst the use of inhaled LABAs are still recommended in asthma guidelines for the resulting improved symptom control,[1] further concerns have been raised, by a large meta-analysis of the pooled results from 19 trials with 33,826 participants, that salmeterol may increase the small risks of asthma deaths and this additional risk is not reduced with the additional use of inhaled steroids (e.g. as with the combination product Fluticasone/salmeterol).[2] This seems to occur because although LABAs relieve asthma symptoms, they also promote bronchial inflammation and sensitivity without warning.[3]
See also
Footnotes
- ↑ British Thoracic Society & Scottish Intercollegiate Guidelines Network (SIGN). British Guideline on the Management of Asthma. Guideline No. 63. Edinburgh:SIGN; 2004. (HTML, Full PDF, Summary PDF)
- ↑ Salpeter S, Buckley N, Ormiston T, Salpeter E (2006). "Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths". Ann Intern Med. 144 (12): 904–12. PMID 16754916. External link in
|title=(help) - ↑ Krishna Ramanujan (2006). "Common asthma inhalers cause up to 80 percent of asthma-related deaths, Cornell and Stanford researchers assert". ChronicalOnline - Cornell University. Unknown parameter
|month=ignored (help); External link in|title=(help)
- Pages with script errors
- CS1 errors: external links
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Beta-adrenergic agonists
- Asthma
- Drugs
- Pulmonology