Rimantadine description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Description
Flumadine® (rimantadine hydrochloride) is a synthetic antiviral drug available as a 100 mg film-coated tablet and as a syrup for oral administration. Each film-coated tablet contains 100 mg of rimantadine hydrochloride plus hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, FD&C Yellow No. 6 Lake and FD&C Yellow No. 6. The film coat contains hydroxypropyl methylcellulose and polyethylene glycol. Each teaspoonful (5 mL) of the syrup contains 50 mg of rimantadine hydrochloride in an aqueous solution containing citric acid, parabens (methyl and propyl), saccharin sodium, sorbitol, D&C Red No. 33 and flavors.
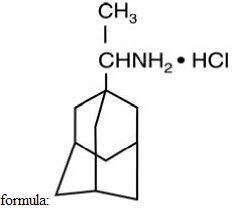
Rimantadine hydrochloride is a white to off-white crystalline powder which is freely soluble in water (50 mg/mL at 20°C). Chemically, rimantadine hydrochloride is alpha-methyltricyclo-[3.3.1.1/3.7]decane-1-methanamine hydrochloride, with an empirical formula of C12H21N•HCI, a molecular weight of 215.77 and the following structural
 |
References
Adapted from the FDA Package Insert.