Retapamulin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Retapamulin is a pleuromutilin antibacterial that is FDA approved for the treatment of impetigo due to Staphylococcus aureus (methicillin-susceptible isolates only) or Streptococcus pyogenes in patients aged 9 months or older. Common adverse reactions include application site irritation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Impetigo
- ALTABAX® is indicated for use in adults for the topical treatment of impetigo (up to 100 cm2 in total area in adults) due to Staphylococcus aureus (methicillin-susceptible isolates only) or Streptococcus pyogenes. Safety in patients younger than 9 months has not been established.
- A thin layer of ALTABAX should be applied to the affected area (up to 100 cm2 in total area in adults) twice daily for 5 days. The treated area may be covered with a sterile bandage or gauze dressing if desired.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Retapamulin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Retapamulin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Impetigo
- ALTABAX® is indicated for use in pediatric patients aged 9 months and older for the topical treatment of impetigo (2% total body surface area in pediatric patients aged 9 months or older) due to Staphylococcus aureus (methicillin-susceptible isolates only) or Streptococcus pyogenes. Safety in patients younger than 9 months has not been established.
- A thin layer of ALTABAX should be applied to the affected area (2% total body surface area in pediatric patients aged 9 months or older) twice daily for 5 days. The treated area may be covered with a sterile bandage or gauze dressing if desired.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Retapamulin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Retapamulin in pediatric patients.
Contraindications
- None.
Warnings
Precautions
- Local Irritation
- In the event of sensitization or severe local irritation from ALTABAX, usage should be discontinued, the ointment wiped off, and appropriate alternative therapy for the infection instituted.
- Not for Systemic or Mucosal Use
- ALTABAX is not intended for ingestion or for oral, intranasal, ophthalmic, or intravaginal use. The efficacy and safety of ALTABAX on mucosal surfaces have not been established. Epistaxis has been reported with the use of ALTABAX on nasal mucosa.
- Potential for Microbial Overgrowth
- The use of antibiotics may promote the selection of nonsusceptible organisms. Should superinfection occur during therapy, appropriate measures should be taken.
- Prescribing ALTABAX in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from the clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
- The safety profile of ALTABAX was assessed in 2,115 adult and pediatric subjects ≥9 months who used at least one dose from a 5-day, twice a day regimen of retapamulin ointment. Control groups included 819 adult and pediatric subjects who used at least one dose of the active control (oral cephalexin), 172 subjects who used an active topical comparator (not available in the US), and 71 subjects who used placebo.
- Adverse events rated by investigators as drug-related occurred in 5.5% (116/2,115) of subjects treated with retapamulin ointment, 6.6% (54/819) of subjects receiving cephalexin, and 2.8% (2/71) of subjects receiving placebo. The most common drug-related adverse events (≥1% of subjects) were application site irritation (1.4%) in the retapamulin group, diarrhea (1.7%) in the cephalexin group, and application site pruritus (1.4%) and application site paresthesia (1.4%) in the placebo group.
- Adults:
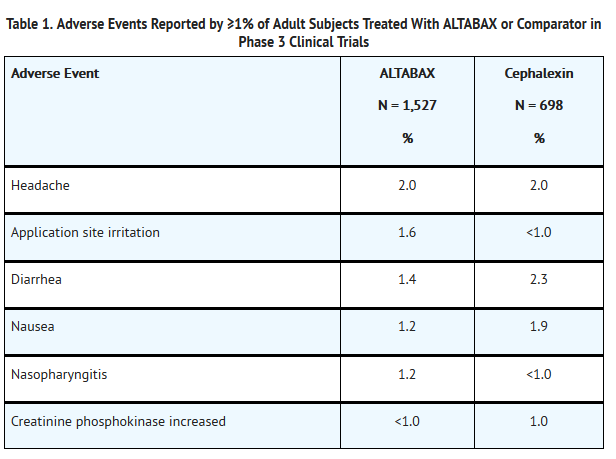
- The adverse events, regardless of attribution, reported in at least 1% of adults (aged 18 years and older) who received ALTABAX or comparator are presented in Table 1.
- Pediatrics:
- The adverse events, regardless of attribution, reported in at least 1% of pediatric subjects aged 9 months to 17 years who received ALTABAX are presented in Table 2.
- Other Adverse Events:
- Application site pain, erythema, and contact dermatitis were reported in less than 1% of subjects in clinical trials.
Postmarketing Experience
- In addition to reports in clinical trials, the following events have been identified during postmarketing use of ALTABAX. Because these events are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General Disorders and Administration Site Conditions
Application site burning.
Immune System Disorders
Hypersensitivity including angioedema.
Drug Interactions
- Coadministration of oral ketoconazole 200 mg twice daily increased retapamulin geometric mean AUC(0-24) and Cmaxby 81% after topical application of retapamulin ointment, 1% on the abraded skin of healthy adult males. Due to low systemic exposure to retapamulin following topical application in adults and pediatric patients aged 2 years and older, dosage adjustments for retapamulin are unnecessary when coadministered with CYP3A4 inhibitors, such as ketoconazole. Based on in vitro P450 inhibition studies and the low systemic exposure observed following topical application of ALTABAX, retapamulin is unlikely to affect the metabolism of other P450 substrates.
- Concomitant administration of retapamulin and CYP3A4 inhibitors, such as ketoconazole, has not been studied in pediatric patients. In pediatric subjects aged 2 to 24 months, systemic exposure of retapamulin was higher compared with subjects aged 2 years and older after topical application. Based on the higher exposure of retapamulin, it is not recommended to coadminister ALTABAX with strong CYP3A4 inhibitors in patients younger than 24 months.
- The effect of concurrent application of ALTABAX and other topical products to the same area of skin has not been studied.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Effects on embryo-fetal development were assessed in pregnant rats given 50, 150, or 450 mg/kg/day by oral gavage on days 6 to 17 postcoitus. Maternal toxicity (decreased body weight gain and food consumption) and developmental toxicity (decreased fetal body weight and delayed skeletal ossification) were evident at doses ≥150 mg/kg/day. There were no treatment-related malformations observed in fetal rats.
- Retapamulin was given as a continuous intravenous infusion to pregnant rabbits at dosages of 2.4, 7.2, or 24 mg/kg/day from day 7 to 19 of gestation. Maternal toxicity (decreased body weight gain, food consumption, and abortions) was demonstrated at dosages ≥7.2 mg/kg/day (8-fold the estimated maximum achievable human exposure, based on AUC, at 7.2 mg/kg/day). There was no treatment-related effect on embryo-fetal development.
- There are no adequate and well-controlled trials in pregnant women. Because animal reproduction studies are not always predictive of human response, ALTABAX should be used in pregnancy only when the potential benefits outweigh the potential risk.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Retapamulin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Retapamulin during labor and delivery.
Nursing Mothers
- It is not known whether retapamulin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ALTABAX is administered to a nursing woman. The safe use of retapamulin during breastfeeding has not been established.
Pediatric Use
- The safety and effectiveness of ALTABAX in the treatment of impetigo have been established in pediatric patients aged 9 months to 17 years. Use of ALTABAX in pediatric patients (9 months to 17 years) is supported by evidence from adequate and well-controlled trials of ALTABAX in which 588 pediatric subjects received at least one dose of retapamulin ointment, 1%. The magnitude of efficacy and the safety profile of ALTABAX in pediatric subjects 9 months and older were similar to those in adults.
- The safety and effectiveness of ALTABAX in pediatric patients younger than 9 months of age have not been established. An open-label clinical trial of topical treatment with ALTABAX (twice daily for 5 days) was conducted in patients aged 2 to 24 months. Plasma samples were obtained from 79 subjects. In these pediatric subjects, systemic exposure of retapamulin was higher compared with subjects aged 2 to 17 years. Furthermore, a higher proportion of pediatric subjects aged 2 to 9 months had measurable concentrations (>0.5 ng/mL) of retapamulin compared with subjects aged 9 to 24 months. The highest levels were seen in subjects aged 2 to 6 months. The use of retapamulin is not indicated in pediatric patients younger than 9 months.
Geriatic Use
- Of the total number of subjects in the adequate and well-controlled trials of ALTABAX, 234 subjects were 65 years of age and older, of whom 114 subjects were 75 years of age and older. No overall differences in effectiveness or safety were observed between these subjects and younger adult subjects.
Gender
There is no FDA guidance on the use of Retapamulin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Retapamulin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Retapamulin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Retapamulin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Retapamulin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Retapamulin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Retapamulin in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Retapamulin in the drug label.
Overdosage
Acute Overdose
- Overdosage with ALTABAX has not been reported. Any signs or symptoms of overdose, either topically or by accidental ingestion, should be treated symptomatically consistent with good clinical practice.
- There is no known antidote for overdoses of ALTABAX.
Chronic Overdose
There is limited information regarding Chronic Overdose of Retapamulin in the drug label.
Pharmacology
Mechanism of Action
- Retapamulin selectively inhibits bacterial protein synthesis by interacting at a site on the 50S subunit of the bacterial ribosome through an interaction that is different from that of other antibiotics. This binding site involves ribosomal protein L3 and is in the region of the ribosomal P site and peptidyl transferase center. By virtue of binding to this site, pleuromutilins inhibit peptidyl transfer, block P-site interactions, and prevent the normal formation of active 50S ribosomal subunits. Retapamulin is bacteriostatic against Staphylococcus aureus and Streptococcus pyogenes at the retapamulin in vitro minimum inhibitory concentration (MIC) for these organisms. At concentrations 1,000x the in vitro MIC, retapamulin is bactericidal against these same organisms. Although cross-resistance between retapamulin and other antibacterial classes (such as clindamycin and oxazolidones) exist, isolates resistant to these classes may be susceptible to retapamulin.
Structure
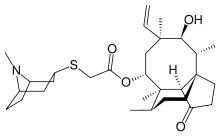
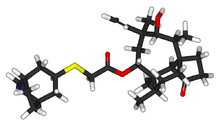
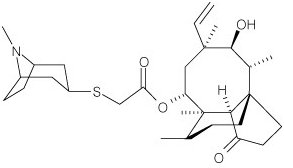
- ALTABAX contains retapamulin, a semisynthetic pleuromutilin antibiotic. The chemical name of retapamulin is acetic acid, [(3-exo)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl]thio]-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3aH-cyclopentacycloocten-8-yl ester. Retapamulin, a white to pale-yellow crystalline solid, has a molecular formula of C30H47NO4S, and a molecular weight of 517.78. The chemical structure is:
- Each gram of ointment for dermatological use contains 10 mg of retapamulin in white petrolatum.
Pharmacodynamics
- In post-hoc analyses of manually over-read 12-lead ECGs from healthy subjects (N = 103), no significant effects on QT/QTc intervals were observed after topical application of retapamulin ointment on intact and abraded skin. Due to the low systemic exposure to retapamulin with topical application, QT prolongation in patients is unlikely.
Pharmacokinetics
- Absorption:
- In a trial of healthy adult subjects, retapamulin ointment, 1% was applied once daily to intact skin (800 cm2 surface area) and to abraded skin (200 cm2 surface area) under occlusion for up to 7 days. Systemic exposure following topical application of retapamulin through intact and abraded skin was low. Three percent of blood samples obtained on Day 1 after topical application to intact skin had measurable retapamulin concentrations (lower limit of quantitation 0.5 ng/mL); thus Cmax values on Day 1 could not be determined. Eighty-two percent of blood samples obtained on Day 7 after topical application to intact skin and 97% and 100% of blood samples obtained after topical application to abraded skin on Days 1 and 7, respectively, had measurable retapamulin concentrations. The median Cmax value in plasma after application to 800 cm2 of intact skin was 3.5 ng/mL on Day 7 (range: 1.2 to 7.8 ng/mL). The median Cmax value in plasma after application to 200 cm2 of abraded skin was 11.7 ng/mL on Day 1 (range: 5.6 to 22.1 ng/mL) and 9.0 ng/mL on Day 7 (range: 6.7 to 12.8 ng/mL).
- Plasma samples were obtained from 380 adult subjects and 136 pediatric subjects (aged 2 to 17 years) who were receiving topical treatment with ALTABAX topically twice daily. Eleven percent had measurable retapamulin concentrations (lower limit of quantitation 0.5 ng/mL), of which the median concentration was 0.8 ng/mL. The maximum measured retapamulin concentration in adults was 10.7 ng/mL and in pediatric subjects (aged 2 to 17 years) was 18.5 ng/mL.
- A single plasma sample was obtained from 79 pediatric subjects (aged 2 to 24 months) who were receiving topical treatment with ALTABAX twice daily. Forty-six percent had measurable retapamulin concentrations (>0.5 ng/mL) compared with 7% in pediatric subjects aged 2 to 17 years. A higher proportion (69%) of pediatric subjects aged 2 to 9 months had measurable concentrations of retapamulin compared with subjects aged 9 to 24 months (32%). Among pediatric subjects aged 2 to 9 months (n = 29), 4 subjects had retapamulin concentrations that were higher (≥26.9 ng/mL) than the maximum concentration observed in pediatric subjects aged 2 to 17 years (18.5 ng/mL). Among pediatric subjects aged 9 to 24 months (n = 50), 1 subject had a retapamulin concentration that was higher (95.1 ng/mL) than the maximum level observed in pediatric subjects aged 2 to 17 years.
- Distribution:
- Retapamulin is approximately 94% bound to human plasma proteins, and the protein binding is independent of concentration. The apparent volume of distribution of retapamulin has not been determined in humans.
- Metabolism:
- In vitro studies with human hepatocytes showed that the main routes of metabolism were mono-oxygenation and di-oxygenation. In vitro studies with human liver microsomes demonstrated that retapamulin is extensively metabolized to numerous metabolites, of which the predominant routes of metabolism were mono-oxygenation and N-demethylation. The major enzyme responsible for metabolism of retapamulin in human liver microsomes was cytochrome P450 3A4 (CYP3A4).
- Elimination:
- Retapamulin elimination in humans has not been investigated due to low systemic exposure after topical application.
Nonclinical Toxicology
- Long-term studies in animals to evaluate carcinogenic potential have not been conducted with retapamulin.
- Retapamulin showed no genotoxicity when evaluated in vitro for gene mutation and/or chromosomal effects in the mouse lymphoma cell assay, in cultured human peripheral blood lymphocytes, or when evaluated in vivo in a rat micronucleus test.
- No evidence of impaired fertility was found in male or female rats given retapamulin 50, 150, or 450 mg/kg/day orally.
Clinical Studies
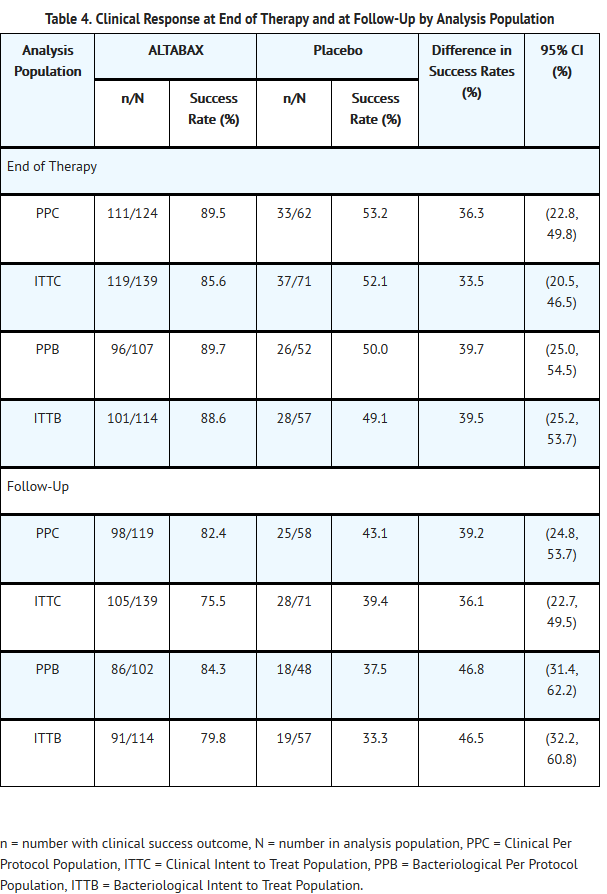
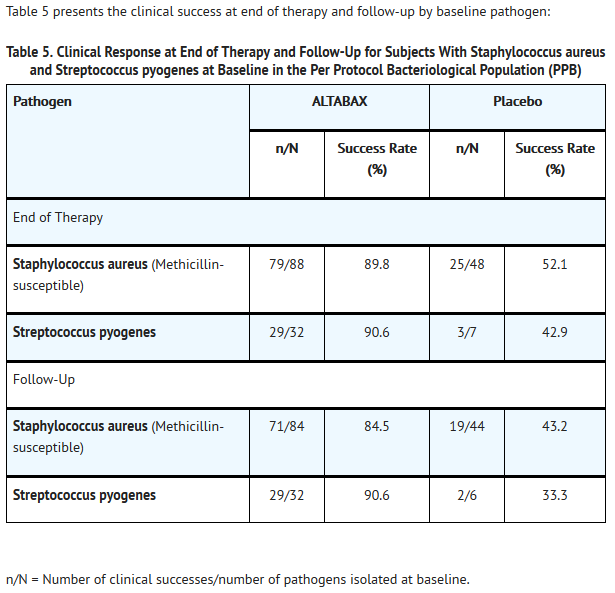
- ALTABAX was evaluated in a placebo-controlled trial that enrolled adult and pediatric subjects aged 9 months and older for treatment of impetigo up to 100 cm2 in total area (up to 10 lesions) or a total body surface area not exceeding 2%. The majority of subjects enrolled (164/210, 78%) were under the age of 13. The trial was a double-blind, randomized, multi-center, parallel-group comparison of the safety of ALTABAX and placebo ointment, both applied twice daily for 5 days. Patients were randomized to ALTABAX or placebo (2:1). Subjects with underlying skin disease (e.g., pre-existing eczematous dermatitis) or skin trauma, with clinical evidence of secondary infection were excluded from these trials. In addition, subjects with any systemic signs and symptoms of infection (such as fever) were excluded from the trial. Clinical success was defined as the absence of treated lesions, or treated lesions had become dry without crusts with or without erythema compared with baseline, or had improved (defined as a decline in the size of the affected area, number of lesions or both) such that no further antimicrobial therapy was required. The intent-to-treat clinical (ITTC) population consisted of all randomized subjects who took at least 1 dose of trial medication. The clinical per protocol (PPC) population included all ITTC subjects who satisfied the inclusion/exclusion criteria and subsequently adhered to the protocol. The intent-to-treat bacteriological (ITTB) population consisted of all randomized subjects who took at least 1 dose of trial medication and had a pathogen identified at trial entry. The bacteriological per protocol (PPB) population included all ITTB subjects who satisfied the inclusion/exclusion criteria and subsequently adhered to the protocol.
- Table 4 presents the results for clinical response at end of therapy (2 days after treatment) and follow-up (9 days after treatment), by analysis population:
- Examination of age and gender subgroups did not identify differences in response to ALTABAX among these groups. The majority of subjects entered into this trial were classified as White/Caucasian or of Asian heritage; when response rates by racial subgroups were viewed across trials, differences in response to ALTABAX were not identified.
How Supplied
- ALTABAX is supplied in 15-, and 30-gram tubes.
- NDC 0007-5180-22 (15 gram tube)
- NDC 0007-5180-25 (30 gram tube)
- Store at 25°C (77°F) with excursions permitted to 15°-30°C (59°-86°F).
Storage
There is limited information regarding Retapamulin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Retapamulin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Retapamulin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients using ALTABAX and/or their guardians should receive the following information and instructions:
- Use ALTABAX as directed by the healthcare practitioner. As with any topical medication, patients and caregivers should wash their hands after application if the hands are not the area for treatment.
- ALTABAX is for external use only. Do not swallow ALTABAX or use it in the eyes, on the mouth or lips, inside the nose, or inside the female genital area.
- The treated area may be covered by a sterile bandage or gauze dressing, if desired. This may also be helpful for infants and young children who accidentally touch or lick the lesion site. A bandage will protect the treated area and avoid accidental transfer of ointment to the eyes or other areas.
- Use the medication for the full time recommended by the healthcare practitioner, even though symptoms may have improved.
- Notify the healthcare practitioner if there is no improvement in symptoms within 3 to 4 days after starting use of ALTABAX.
- ALTABAX may cause reactions at the site of application of the ointment. Inform the healthcare practitioner if the area of application worsens in irritation, redness, itching, burning, swelling, blistering, or oozing.
Precautions with Alcohol
- Alcohol-Retapamulin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ALTABAX®[1]
Look-Alike Drug Names
There is limited information regarding Retapamulin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Retapamulin
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Retapamulin |Label Name=Retapamulin06.png
}}
{{#subobject:
|Label Page=Retapamulin |Label Name=Retapamulin07.png
}}