Renal tubular acidosis pathophysiology
|
Renal tubular acidosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Renal tubular acidosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Renal tubular acidosis pathophysiology |
|
Risk calculators and risk factors for Renal tubular acidosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] ; Associate Editor(s)-in-Chief: Aditya Ganti M.B.B.S. [2] Syed Ahsan Hussain, M.D.[3] Jogeet Singh Sekhon, M.D. [4]
Overview
Kidneys reabsorb the filtered bicarbonate and excrete acid to maintain acid-base balance. HCO3 reabsorption is facilitated by Na-H and proton pumps. Collecting tubules serve the function of excretion of acid. Renal tubular acidosis is described as any one of a number of disorders, in which either of above buffering mechanism is impaired. Potassium is the most common electrolyte abnormality that can be noticed with renal tubular acidosis. It can be either hypokalemic renal tubular acidosis or hyperkalemic renal tubular acidosis. Mode of inheritance can autosomal dominant or recessive depending upon type of disease abnormality, Common genes involved include ATP6V1B1, ATP6V0A4, SLC4A1.
Pathophysiology
Normal Physiology of Acid-Base balance
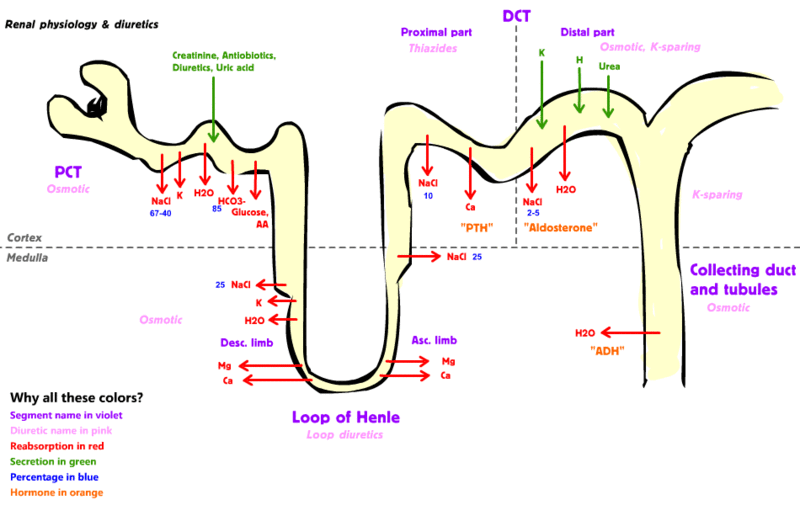
- Normally kidneys reabsorb the filtered bicarbonate and excrete acid to maintain acid-base balance.[1][2]
- HCO3 reabsorption is facilitated by Na-H and proton pumps.
- Na-H reabsorbs about 80-90% of the filtered HCO3 at the proximal tubule.
- Proton pumps (H-ATPase and H-K ATPase) in the distal nephron reabsorbs remaining 10 percent of HCO3.
- There is no HCO3 in the final urine.
- Collecting tubules serve the function of excretion of acid.[3][4]
- Hydrogen ions need a buffer to get excreted.
- The principal buffers in the urine are ammonia and phosphate.
- Acidosis stimulates ammonia production in renal tubules.
- While ammonia can freely diffuse across membranes, ammonium cannot.
- The secretion of hydrogen ions into the tubular lumen trap ammonia as ammonium which can easily flush out along with .
- Increased production of ammonium is required in cases of acidosis to maintain near-normal balance.
Potassium abnoralities
- Potassium is the most common electrolyte abnormality that can be noticed with renal tubular acidosis.[5][6]
- It can be either hypokalemic renal tubular acidosis or hyperkalemic renal tubular acidosis.
- Almost all of the filtered potassium is reabsorbed passively in the proximal tubule and loop of Henle.
- The potassium excreted in the urine is derived from secretion into the tubular lumen by cells in the distal nephron.
- Distal potassium secretion is primarily influenced by two factors, both promote sodium reabsorption:
- Depending upon the site of the defect and the mechanism responsible for the various forms of renal tubular acidosis, can result in hypokalemia or hyperkalemia:
- Hypokalemia frequently develops in patients with distal renal tubular acidosis.
- Usually improves with alkali therapy in contrast to to hypokalemia in proximal renal tubular acidosis which is exacerbated by alkali therapy.
- Hyperkalemia occurs frequently with hypoaldosteronism (type 4 ) and in patients with other defects in distal nephron sodium reabsorption (voltage-dependent renal tubular acidosis).
- Hypokalemic renal tubular acidosis
- Type 1 renal tubular acidosis
- Type 2 renal tubular acidosis
- Hyperkalemic renal tubular acidosis
- Type 4 renal tubular acidosis
- Voltage-dependent renal tubular acidosis
Distal renal tubular acidosis
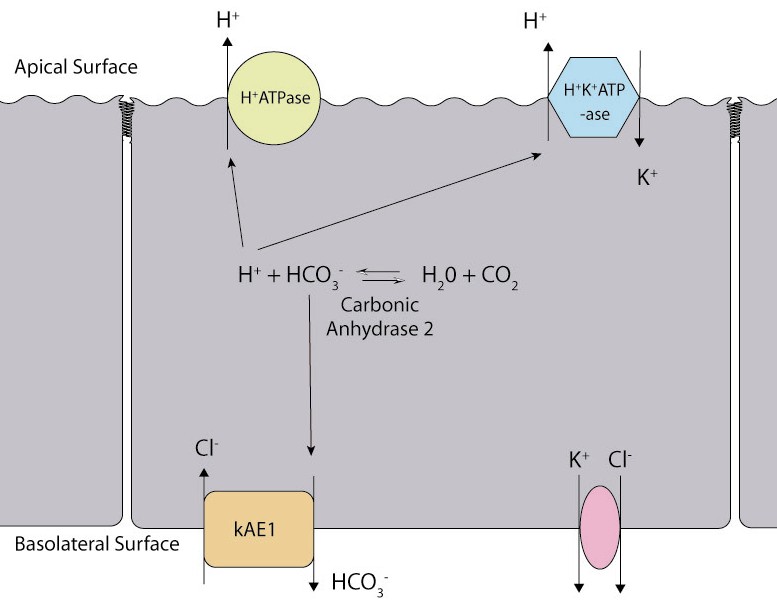
- Type 1 renal tubular acidosis is characterized by impaired hydrogen ion secretion in the distal nephron.
- If left untreated, it results in progressive hydrogen ion retention leading to normal anion gap metabolic acidosis.
- Type 1 renal tubular acidosis results in hypokalemia.
- Impaired hydrogen ion secretion in patients with distal renal tubular acidosis can be caused by several defects:
- Decreased net activity of the proton pump.
- Increased hydrogen ion permeability of the luminal membrane.
Incomplete distal renal tubular acidosis
- Incomplete distal renal tubular acidosis is a variant in which patients cannot acidify their urine, resulting in a urine pH that is persistently 5.5 or higher.
- The low rate of citrate excretion and relatively high rate of ammonium excretion are believed to the inciting factor for a reduced intracellular pH within the cells of the proximal tubule .
- Persistent hypo-citraturia is a consistent feature of incomplete renal tubular acidosis.
Proximal renal tubular acidosis
- Proximal renal tubular acidosis is characterized by a decrease in re-absorption of bicarbonate in the proximal tubule.
- Within proximal tubule cells, hydrogen ions and bicarbonate are generated from carbonic acid.
- Na-K-ATPase pump facilitates the movement of sodium down the electrochemical concentration gradient from the lumen into the cells.
- This concentration gradient drives hydrogen ions in the opposite direction which is counter balanced by bicarbonate ions via a sodium-bicarbonate cotransporter.
- The net effect of this process is that, for every hydrogen ion molecule secreted into the lumen, a bicarbonate molecule enters the peritubular capillary.
- Abnormalities of one or more of these proximal tubule transporters, pumps, or enzymes can impair sodium bicarbonate reabsorption and cause the bicarbonate wasting found in proximal renal tubular acidosis.
Hyperkalemic renal tubular acidosis
Most common causes of hyperkalemic renal tubular acidosis include:
- Voltage-dependent renal tubular acidosis
Voltage-dependent renal tubular acidosis
- Voltage-dependent renal tubular acidosis occurs with markedly reduced distal sodium delivery
- Inherited or acquired defects in sodium reabsorption by the principal cells.
- Most common etiology include
- Hypovolemia
- UTI
- Lupus nephritis
- Sickle cell disease
- Medications
Hypoaldosteronism
- Aldosterone deficiency or resistance produces a hyperkalemic renal tubular acidosis.[7]
- Type 4 renal tubular acidosis is associated with milder
- The acidification defect is primarily due to a reduced rate of proton secretion.
- Thus, when very little buffer (ammonia [NH3] and/or phosphate) is present in the renal tubule lumen, the pH can be appropriately reduced below pH 5.5 (but the quantity of excreted acid is relatively low).
- By contrast, when greater amounts of buffer are present in the distal tubule, the impaired rate of hydrogen ion secretion produces a faster rise in the urine pH above 5.5 than is seen in normal subjects.
- In either case, the reduction in net acid excretion results in metabolic acidosis.
Patients with hyperkalemia usually have lower urine ammonia levels and, therefore, a more acidic urine. The defect produced by the various forms of voltage-dependent distal RTA discussed above is similar but generally more severe. In that disorder, the urine pH often cannot be reduced below 5.5 but can also vary with the rate of urine ammonia excretion.
| Type of RTA | Primary defect | Plasma HCO3 mEq/L | Urine pH | Plasma potassium | Urine anion gap | Urine calcium/creatinine ratio | Risk for nephrolithiasis |
|---|---|---|---|---|---|---|---|
| RTA type 1 | Impaired distal acidification | < 10 | >5.3 | Hypokalemic | Positive | ↑ | ↑ |
| RTA Type 2 | Reduced proximal HCO3 reabsorption. | 12 to 20 | <5.3 | Hypokalemic | Negative | Normal | - |
| RTA type 4 | Decreased aldosterone secretion
Aldosterone resistance |
>17 | Variable | Hyperkalemia | Positive | Normal | - |
| Voltage-dependent RTA | Reduced sodium reabsorption | >17 | Variable | Hyperkalemia | Positive | Normal | - |
Associated Conditions
Common conditions associated with renal tubular acidosis include:
- Congenital hypoaldosteronism
- Primary adrenal insufficiency
- Wilson disease
- Sarcoidosis
- Autoimmune disorders
Genetics
- DRTA may be inherited as an autosomal dominant or recessive trait.[8]
- Autosomal recessive DRTA often presents in infancy
- Autosomal dominant DRTA may not present until adolescence or young adulthood.
- Mutations in the genes encoding carbonic anhydrase II, kidney anion exchanger 1 (kAE1), and subunits of the renal proton pump (H+‐ATPase) have been identified in patients with DRTA.
- Genetically transmitted PRTAs include autosomal dominant and recessive forms.
- PRTA (with ocular abnormalities) may be caused by inactivating mutations in the Na/HCO3 cotransporter gene (SLC4A4).
- PRTA may also be associated with other genetically transmitted disorders, such as osteopetrosis with carbonic anhydrase II deficiency.
- Inherited defects leading to Type 4 RTA are due to aldosterone deficiency or resistance.
- Congenital adrenal hyperplasia with salt wasting
- Isolated hypoaldosteronism
- Pseudohypoaldosteronism (defect at the aldosterone receptor level)
| Type of RTA | Gene | Gene location | Protein | |
|---|---|---|---|---|
| Type 1 (distal) RTA | Autosomal recessive with deafness | ATP6V1B1 | 2p13 | B1 subunit of H-ATPase |
| Autosomal recessive without deafness | ATP6V0A4 | 7q33-q34 | a4 subunit of H-ATPase | |
| Autosomal dominant | SLC4A1 | 17q21-q22 | Chloride-bicarbonate exchanger | |
| Type 2 (proximal) RTA | Autosomal recessive | SLC4A4 | 4q21 | Sodium bicarbonate cotransporter |
| Type 3 (mixed) RTA | Autosomal recessive | Carbonic anhydrase II | 8q22 | Carbonic anhydrase II |
References
- ↑ Soleimani M, Burnham CE (February 2000). "Physiologic and molecular aspects of the Na+:HCO3- cotransporter in health and disease processes". Kidney Int. 57 (2): 371–84. doi:10.1046/j.1523-1755.2000.00857.x. PMID 10652014.
- ↑ Rodríguez Soriano J (August 2002). "Renal tubular acidosis: the clinical entity". J. Am. Soc. Nephrol. 13 (8): 2160–70. PMID 12138150.
- ↑ Wagner CA, Geibel JP (2002). "Acid-base transport in the collecting duct". J. Nephrol. 15 Suppl 5: S112–27. PMID 12027210.
- ↑ Stanton BA (November 1989). "Renal potassium transport: morphological and functional adaptations". Am. J. Physiol. 257 (5 Pt 2): R989–97. doi:10.1152/ajpregu.1989.257.5.R989. PMID 2686470.
- ↑ Pereira PC, Miranda DM, Oliveira EA, Silva AC (March 2009). "Molecular pathophysiology of renal tubular acidosis". Curr. Genomics. 10 (1): 51–9. doi:10.2174/138920209787581262. PMC 2699831. PMID 19721811.
- ↑ Szylman, Pedro; Better, Ori S.; Chaimowitz, Cidio; Rosler, Ariel (1976). "Role of Hyperkalemia in the Metabolic Acidosis of Isolated Hypoaldosteronism". New England Journal of Medicine. 294 (7): 361–365. doi:10.1056/NEJM197602122940703. ISSN 0028-4793.
- ↑ Szylman, Pedro; Better, Ori S.; Chaimowitz, Cidio; Rosler, Ariel (1976). "Role of Hyperkalemia in the Metabolic Acidosis of Isolated Hypoaldosteronism". New England Journal of Medicine. 294 (7): 361–365. doi:10.1056/NEJM197602122940703. ISSN 0028-4793.
- ↑ Karet FE (August 2002). "Inherited distal renal tubular acidosis". J. Am. Soc. Nephrol. 13 (8): 2178–84. PMID 12138152.