Piperacillin sodium clinical pharmacology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Clinical Pharmacology
Intravenous Administration:
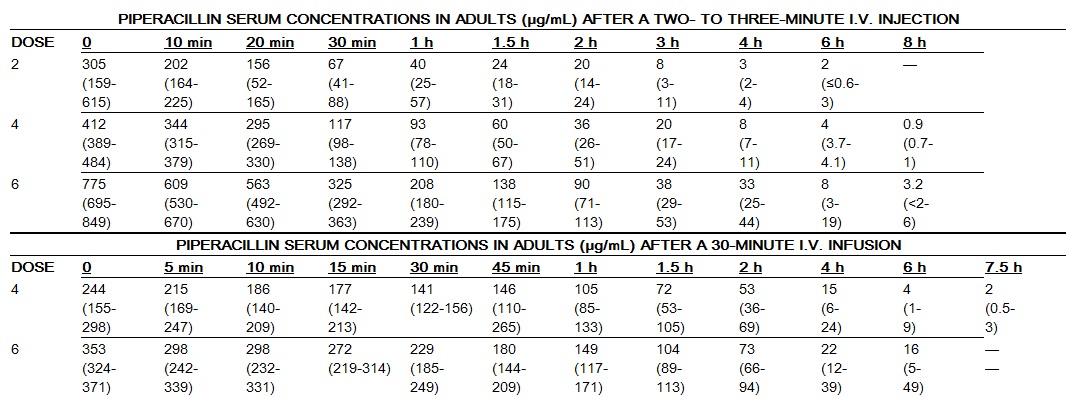
In healthy adult volunteers, mean serum piperacillin concentrations immediately after a two‑to three‑minute intravenous injection of 2, 4, or 6 g were 305, 412, and 775 μg/mL, respectively. Serum concentrations lack dose proportionality.
A 30-minute infusion of 6 g every 6 h gave, on the fourth day, a mean peak serum concentration of 420 μg/mL.
Intramuscular Administration:
PIPRACIL is rapidly absorbed after intramuscular injection. In healthy volunteers, the mean peak serum concentration occurs approximately 30 minutes after a single dose of 2 g and is about 36 μg/mL. The oral administration of 1 g probenecid before injection produces an increase in piperacillin peak serum level of about 30%. The area under the curve (AUC) is increased by approximately 60%.
Pharmacokinetics:
PIPRACIL is not absorbed when given orally. Peak serum concentrations are attained approximately 30 minutes after intramuscular injections and immediately after completion of intravenous injection or infusion. The serum half-life in healthy volunteers ranges from 36 minutes to one hour and 12 minutes. The mean elimination half-life of PIPRACIL in healthy adult volunteers is 54 minutes following administration of 2 g and 63 minutes following 6 g. As with other penicillins, PIPRACIL is eliminated primarily by glomerular filtration and tubular secretion; it is excreted rapidly as unchanged drug in high concentrations in the urine. Approximately 60% to 80% of the administered dose is excreted in the urine in the first 24 hours. Piperacillin urine concentrations, determined by microbioassay, are as high as 14,100 μg/mL following a 6-g intravenous dose and 8,500 μg/mL following a 4-g intravenous dose. These urine drug concentrations remain well above 1,000 μg/mL throughout the dosing interval.
Distribution:
PIPRACIL binding to human serum proteins is 16%. The drug is widely distributed in human tissues and body fluids, including bone, prostate, and heart, and reaches high concentrations in bile. After a 4-g bolus injection, maximum biliary concentrations average 3,205 μg/mL. It penetrates into the cerebrospinal fluid in the presence of inflamed meninges.
Special Populations:
Renal Insufficiency: The elimination half-life is increased twofold in mild to moderate renal impairment and fivefold to sixfold in severe impairment. Because PIPRACIL is excreted by the biliary route as well as by the renal route, it can be used safely in appropriate dosage in patients with severely restricted kidney function. (See DOSAGE AND ADMINISTRATION.)
Pediatric Patients: After intravenous administration of 50 mg/kg (5-minute infusion) in neonates, the mean plasma concentration of piperacillin extrapolated to time zero was 141 μg/mL, and the apparent volume of distribution averaged 101 mL/kg.
In premature neonates, the mean elimination half-life has been reported to range from 147 to 258 minutes following administration of a single intravenous dose of 75 mg/kg, the half‑life decreasing with increasing postnatal age. The changes in half-life appeared to be caused by an immature renal system during the first weeks of life. In one study in neonates, the mean elimination half-life ranged from 127 to 217 minutes following a single intravenous dose of 50 mg/kg. As in premature neonates, the half-life in neonates decreased with increasing postnatal age. The mean total body clearance in neonates has been reported to range from 32 to 41 mL/min/1.73 m2 after an intravenous dose of 50 mg/kg.
Following administration of an intravenous dose of 50 mg/kg in older pediatric patients (from 1 month up to 15 years of age), the mean elimination half-life has been reported to range from 31 to 37 minutes, and the mean total body clearance has been reported to range from 124 to 160 mL/min/1.73 m2.
As in adults, PIPRACIL elimination tends to be prolonged in pediatric patients with renal impairment. In one study in pediatric patients (age range, 3.3 to 14.3 years), the mean elimination half-life in patients with decreased renal function was approximately 60 minutes versus 37 minutes in patients with normal renal function. The elimination half-life has been reported to range from 3.5 to 14 hours in neonates with severe renal impairment.
Pharmacokinetic data have indicated that among pediatric patients below 12 years of age, those with cystic fibrosis have increased bioavailability, lower serum concentrations, and increased total body clearance of piperacillin compared to young, healthy pediatric volunteers under 12 years of age.[1]
References
- ↑ "PIPRACIL (PIPERACILLIN SODIUM) INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION [WYETH PHARMACEUTICALS, INC.]". Text " accessdate" ignored (help)
Adapted from the FDA Package Insert.