Palonosetron hydrochloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Palonosetron hydrochloride is an antiemetic that is FDA approved for the prophylaxis of chemotherapy-Induced nausea and vomiting. Common adverse reactions include bradyarrhythmia,constipation,headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Prevention of Chemotherapy-Induced Nausea and Vomiting
ALOXI Capsules are indicated for:
- Moderately emetogenic cancer chemotherapy - prevention of acute nausea and vomiting associated with initial and repeat courses.
Dosage
- One 0.5 mg capsule administered approximately one hour prior to the start of chemotherapy. ALOXI can be taken with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Palonosetron hydrochloride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Palonosetron hydrochloride in adult patients.
DOSAGE FORMS AND STRENGTHS
- Capsules, 0.5 mg
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Palonosetron hydrochloride in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Palonosetron hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Palonosetron hydrochloride in pediatric patients.
Contraindications
- ALOXI is contraindicated in patients known to have hypersensitivity to the drug or any of its components.
Warnings
Hypersensitivity
Hypersensitivity reactions may occur in patients who have exhibited hypersensitivity to other 5-HT3 receptor antagonists. Hypersensitivity reactions have been very rarely reported post-marketing for intravenous palonosetron: dyspnea, bronchospasm, swelling/edema, erythema, pruritus, rash, urticaria. No hypersensitivity reactions have been reported for oral palonosetron.
Serotonin Syndrome
- The development of serotonin syndrome has been reported with 5-HT3 receptor antagonists. Most reports have been associated with concomitant use of serotonergic drugs (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors, mirtazapine, fentanyl, lithium, tramadol, and intravenous methylene blue). Some of the reported cases were fatal. Serotonin syndrome occurring with overdose of another 5-HT3 receptor antagonist alone has also been reported. The majority of reports of serotonin syndrome related to 5-HT3 receptor antagonist use occurred in a post-anesthesia care unit or an infusion center.
- Symptoms associated with serotonin syndrome may include the following combination of signs and symptoms: mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g.,tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, with or without gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome, especially with concomitant use of ALOXI and other serotonergic drugs. If symptoms of serotonin syndrome occur, discontinue ALOXI and initiate supportive treatment. Patients should be informed of the increased risk of serotonin syndrome, especially if ALOXI is used concomitantly with other serotonergic drugs.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In clinical trials for the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy, 693 adult patients received oral palonosetron in doses ranging from 0.25 mg to 0.75 mg. Following is a listing of drug related adverse reactions reported by ≥ 2% of patients from two clinical trials.

The infrequently reported adverse reactions listed below, assessed by investigators as treatment-related or causality unknown/missing, occurred following administration of ALOXI Capsules to adult patients receiving concomitant cancer chemotherapy. Of these adverse events, fatigue (incidence 1%), was the only adverse event reported at an incidence of ≥1%. In general, adverse reactions were similar between oral and I.V. formulations.
- Blood and Lymphatic System: <1%: anemia.
- Cardiovascular: <1%: hypertension, transient arrhythmia, first degree atrioventricular block, second degree atrioventricular block, QTc prolongation.
- Hearing and Labyrinth: <1%: motion sickness.
- Eye: <1%: eye swelling.
- Infections: <1%: sinusitis.
- Liver: <1%: transient, asymptomatic increases in bilirubin.
- Nutrition: <1%: anorexia.
- Musculoskeletal: <1%: joint stiffness,myalgia, pain in extremity.
- Nervous System: <1%: postural dizziness, dysgeusia.
- Psychiatric: <1%: insomnia.
- Very rare cases (<1/10,000) of hypersensitivity reactions have been reported for I.V. ALOXI from post-marketing experience.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Palonosetron hydrochloride in the drug label.
Drug Interactions
- Palonosetron is eliminated from the body through both renal excretion and metabolic pathways with the latter mediated via multiple CYP enzymes. Further in vitro studies indicated that palonosetron is not an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1 and CYP3A4/5 (CYP2C19 was not investigated) nor does it induce the activity of CYP1A2, CYP2D6, or CYP3A4/5. Therefore, the potential for clinically significant drug interactions with palonosetron appears to be low.
- Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor antagonists and other serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs).
- A study in healthy volunteers involving single-dose I.V. palonosetron (0.75 mg) and steady state oral metoclopramide (10 mg four times daily) demonstrated no significant pharmacokinetic interaction.
- Concomitant administration of an antacid (Maalox® liquid 30 mL) had no effect on the oral absorption or pharmacokinetics of a single capsule of palonosetron 0.75 mg in healthy subjects.
- In controlled clinical trials, ALOXI Capsules have been safely administered with chemotherapeutic agents, systemic corticosteroids, analgesics, and drugs for gastrointestinal disorders including function gastrointestinal disorders, acid-related disorders, and antiemetics/anti nauseants.
- Palonosetron did not inhibit the antitumor activity of the five chemotherapeutic agents tested (cisplatin, cyclophosphamide, cytarabine, doxorubicin and mitomycin C) in murine tumor models.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Teratogenic effects
Pregnancy Category B.
- Reproduction studies have been performed in rats at oral doses up to 60 mg/kg/day (921 times the recommended human oral dose based on body surface area) and rabbits at oral doses up to 60 mg/kg/day (1841 times the recommended human oral dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to palonosetron. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, palonosetron should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Palonosetron hydrochloride in women who are pregnant.
Labor and Delivery
- Palonosetron has not been administered to patients undergoing labor and delivery, so its effects on the mother or child are unknown.
Nursing Mothers
- It is not known whether palonosetron is excreted in human milk.
- Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants and the potential for tumorigenicity shown for palonosetron in the rat carcinogenicity study, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in patients below the age of 18 years have not been established.
Geriatic Use
- Of the total number of adult cancer patients in a pivotal study of oral palonosetron, 181 were 65 years of age and over. The number of geriatric patients receiving 0.5 mg palonosetron was insufficient to draw any efficacy or safety conclusions.
- In a cross-study comparison, after a single oral dose (0.75 mg) the systemic exposure of palonosetron (AUC) was similar, but mean Cmax was 15% lower in healthy elderly subjects ≥65 years of age compared with the subjects < 65 years of age. No dose adjustment is required for geriatric patients.
Gender
- Although a single dose of 0.5 mg ALOXI Capsule was associated with a 26- 35% higher systemic exposure in female subjects than in male subjects, dosage adjustment is not necessary based on gender.
Race
- Oral pharmacokinetics of palonosetron were characterized in thirty-two healthy Japanese male subjects using solution over the dose range of 3-90 µg/kg. The apparent total body clearance was 26% higher in Japanese males than in white males based on a cross-study comparison; however, no dose adjustment is necessary. The pharmacokinetics of palonosetron in other races have not been adequately characterized.
Renal Impairment
- Mild to moderate renal impairment does not significantly affect palonosetron pharmacokinetic parameters. Total systemic exposure to intravenous ALOXI increased by approximately 28% in severe renal impairment relative to healthy subjects. Dosage adjustment is not necessary in patients with mild to severe renal impairment. The pharmacokinetics of palonosetron have not been studied in subjects with end-stage renal disease.
Hepatic Impairment
- Hepatic impairment does not significantly affect total body clearance of intravenous palonosetron compared to the healthy subjects. Dosage adjustment is not necessary in patients with any degree of hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Palonosetron hydrochloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Palonosetron hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Palonosetron hydrochloride in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Palonosetron hydrochloride in the drug label.
Overdosage
- There is no known antidote to ALOXI. Overdose should be managed with supportive care.
- Thirty-three adult cancer patients were administered oral palonosetron at a dose of 90 µg/kg (equivalent to 6 mg fixed dose) as part of a dose ranging study. This is approximately 12 times the recommended oral dose of 0.5 mg. This dose group had a similar incidence of adverse events compared to the other dose groups and no dose response effects were observed.
- Dialysis studies have not been performed, however, due to the large volume of distribution, dialysis is unlikely to be an effective treatment for palonosetron overdose. A single oral dose of palonosetron at 500 mg/kg in rats and 100 mg/kg in dogs (7673 and 5115 times the recommended human oral dose, respectively, based on body surface area) was lethal. The major signs of toxicity included convulsions, labored breathing, and salivation.
Pharmacology
Mechanism of Action
- Palonosetron is a 5-HT3 receptor antagonist with a strong binding affinity for this receptor and little or no affinity for other receptors.
- Cancer chemotherapy may be associated with a high incidence of nausea and vomiting, particularly when certain agents, such as cisplatin, are used. 5- HT3 receptors are located on the nerve terminals of the vagus in the periphery and centrally in the chemoreceptor trigger zone of the area postrema. It is thought that chemotherapeutic agents produce nausea and vomiting by releasing serotonin from the enterochromaffin cells of the small intestine and that the released serotonin then activates 5-HT3 receptors located on vagal afferents to initiate the vomiting reflex.
Structure
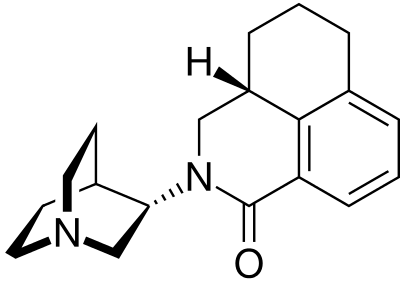
- ALOXI (palonosetron HCl) Capsules is an antiemetic and antinauseant agent. It is a serotonin subtype 3 (5-HT3) receptor antagonist with a strong binding affinity for this receptor. Chemically, palonosetron hydrochloride is: (3aS)-2-[(S)-1-Azabicyclo[2.2.2]oct-3-yl]-2,3,3a,4,5,6-hexahydro-1-oxo-1H-benz[de]isoquinoline hydrochloride. The empirical formula is C19H24N2O.HCl, with a molecular weight of 332.87. Palonosetron hydrochloride exists as a single isomer and has the following structural formula:

- Palonosetron hydrochloride is a white to off-white crystalline powder. It is freely soluble in water, soluble in propylene glycol, and slightly soluble in ethanol and 2-propanol.
- Each light beige opaque soft gelatin ALOXI Capsule contains 0.56 mg of palonosetron HCl equivalent to palonosetron 0.5 mg. Inactive ingredients are: mono- and di-glycerides of capryl/capric acid, glycerin, polyglyceryl oleate, water, and butylated hydroxyanisole.
Pharmacodynamics
- In non-clinical studies palonosetron possesses the ability to block ion channels involved in ventricular de- and re-polarization and to prolong action potential duration.
- The effect of palonosetron on QTc interval was evaluated in a double blind, randomized, parallel, placebo and positive (moxifloxicin) controlled trial in adult men and women. The objective was to evaluate the ECG effects of intravenously administered palonosetron at single doses of 0.25 mg, 0.75 mg or 2.25 mg in 221 healthy subjects. The study demonstrated no significant effect on any ECG interval including QTc duration (cardiac repolarization) at doses up to 2.25 mg.
- Clinical trials revealed that oral palonosetron had comparable effects on blood pressure, heart rate, and ECG parameters as intravenous palonosetron.
Pharmacokinetics
Absorption
- Following oral administration, palonosetron is well absorbed with its absolute bioavailability reaching 97%. After single oral doses using buffered solution mean maximum palonosetron concentrations (Cmax) and area under the concentration-time curve (AUC0-∞) were dose proportional over the dose range of 3.0 to 80 µg/kg in healthy subjects.
- In 36 healthy male and female subjects given a single oral dose of ALOXI Capsules 0.5 mg, maximum plasma palonosetron concentration (Cmax) was 0.81±1.66 ng/mL (mean ± SD) and time to maximum concentration (Tmax) was 5.1± 1.7 hours. In female subjects (n=18), the mean AUC was 35% higher and the mean Cmax was 26% higher than in male subjects (n=18).
- In 12 cancer patients given a single oral dose of palonosetron 0.5 mg one hour prior to chemotherapy, Cmax was 0.93±0.34 ng/mL and Tmax was 5.1± 5.9 hours. The AUC was 30% higher in cancer patients than in healthy subjects. The mean PK parameters after a single oral dose of 0.5 mg palonosetron are compared between healthy subjects and cancer patients (Table 2).

- A high fat meal did not affect the Cmax and AUC of oral palonosetron. Therefore, ALOXI Capsules may be taken without regard to meals.
Distribution
- Palonosetron has a volume of distribution of approximately 8.3 ± 2.5 L/kg. Approximately 62% of palonosetron is bound to plasma proteins.
Metabolism
- Palonosetron is eliminated by multiple routes with approximately 50% metabolized to form two primary metabolites: N-oxide-palonosetron and 6-S- hydroxy-palonosetron. These metabolites each have less than 1% of the 5-HT3 receptor antagonist activity of palonosetron. In vitro metabolism studies have suggested that CYP2D6 and to a lesser extent, CYP3A4 and CYP1A2 are involved in the metabolism of palonosetron. However, clinical pharmacokinetic parameters are not significantly different between poor and extensive metabolizers of CYP2D6 substrates.
Elimination
- Following administration of a single oral 0.75 mg dose of [14C]- palonosetron to six healthy subjects, 85% to 93% of the total radioactivity was excreted in urine, and 5% to 8% was eliminated in feces. The amount of unchanged palonosetron excreted in the urine represented approximately 40% of the administered dose. In healthy subjects given ALOXI Capsules 0.5 mg, the terminal elimination half-life (t½) of palonosetron was 37 ± 12 hours (mean ± SD), and in cancer patients, t½ was 48 ± 19 hours. After a single- dose of approximately 0.75 mg intravenous palonosetron, the total body clearance of palonosetron in healthy subjects was 160 ± 35 mL/h/kg (mean ± SD) and renal clearance was 66.5 ± 18.2 mL/h/kg.
Nonclinical Toxicology
Carciogenesis, Mutagenesis, Impairment of Fertility
- In a 104-week carcinogenicity study in CD-1 mice, animals were treated with oral doses of palonosetron at 10, 30 and 60 mg/kg/day. Treatment with palonosetron was not tumorigenic. The highest tested dose produced a systemic exposure to palonosetron (Plasma AUC) of about 90 to 173 times the human exposure (AUC= 49.7 ng·h/mL) at the recommended oral dose of 0.5 mg. In a 104-week carcinogenicity study in Sprague-Dawley rats, male and female rats were treated with oral doses of 15, 30 and 60 mg/kg/day and 15, 45 and 90 mg/kg/day, respectively. The highest doses produced a systemic exposure to palonosetron (Plasma AUC) of 82 and 185 times the human exposure at the recommended dose. Treatment with palonosetron produced increased incidences of adrenal benign pheochromocytoma and combined benign and malignant pheochromocytoma, increased incidences of pancreatic Islet cell adenoma and combined adenoma and carcinoma and pituitary adenoma in male rats. In female rats, it produced hepatocellular adenoma and carcinoma and increased the incidences of thyroid C-cell adenoma and combined adenoma and carcinoma.
- Palonosetron was not genotoxic in the Ames test, the Chinese hamster ovarian cell (CHO/HGPRT) forward mutation test, the ex vivo hepatocyte unscheduled DNA synthesis (UDS) test or the mouse micronucleus test. It was, however, positive for clastogenic effects in the Chinese hamster ovarian (CHO) cell chromosomal aberration test.
- Palonosetron at oral doses up to 60 mg/kg/day (about 921 times the recommended human oral dose based on body surface area) was found to have no effect on fertility and reproductive performance of male and female rats.
Clinical Studies
- Study 1 was a multicenter, randomized, double-blind active control clinical trial of 635 patients set to receive moderately emetogenic cancer chemotherapy. A single-dose of 0.25 mg, 0.5 mg, or 0.75 mg oral ALOXI Capsules given one hour prior to moderately emetogenic chemotherapy was compared to a single-dose of 0.25 mg I.V. ALOXI given 30 minutes prior to chemotherapy. Patients were randomized to either dexamethasone or placebo in addition to their assigned treatment. The majority of patients in the study were women (73%), white (69%), and naïve to previous chemotherapy (59%). The primary efficacy endpoint was Complete Response (no emetic episodes and no rescue medication) assessed in the acute phase (0-24 hours). A key secondary efficacy endpoint was Complete Response assessed in the delayed phase (24-120 hours). Other secondary endpoints included Complete Response for the acute plus delayed phases (0-120 hours) and No Nausea for the acute and delayed phases.
- Efficacy was based on demonstrating non-inferiority of oral palonosetron doses compared to the approved I.V. formulation. Non- inferiority criteria were met if the lower bound of the two-sided 98.3% confidence interval for the difference in complete response rates of oral palonosetron dose minus approved I.V. formulation was larger than -15%. The non-inferiority margin was 15%.
Efficacy Results
- As shown in Table 3, ALOXI Capsules 0.5 mg demonstrated non- inferiority to the active comparator during the 0 to 24 hour time interval; however, for the 24 to 120 hour time period, non-inferiority was not shown. The additional two oral palonosetron dose levels showed similar results.

- To adjust for multiplicity of treatment groups, a lower-bound of a two-sided 98.3% confidence interval was used to compare to -15%, the negative value of the non-inferiority margin.
- As indicated in the data above, analysis of the key secondary endpoint showed that a single dose of ALOXI Capsules 0.5 mg was numerically similar to a single dose of I.V. ALOXI 0.25 mg, however, statistical non-inferiority was not demonstrated. For ALOXI Capsules 0.5 mg versus I.V. ALOXI 0.25 mg, the proportion of patients with complete response at 0-120 hours was 58.8% versus 59.3%, respectively. The proportions of patients with no nausea at 0-24 and 24-120 hours were also numerically similar between oral and I.V. doses.
- Study 2 was a multicenter, open label, repeat cycle study performed to evaluate the safety and efficacy of single dose oral ALOXI Capsules 0.75 mg in cancer patients receiving moderately emetogenic chemotherapy. An ALOXI Capsule was given to 217 cancer patients in 654 chemotherapy cycles one hour before the start of chemotherapy. Approximately 74% of patients also received single dose oral or intravenous dexamethasone 30 minutes before chemotherapy. Complete Response was not formally evaluated for the repeat cycle application. However, in general the antiemetic effect for the 0-24 hour interval was similar throughout the consecutively repeated cycles.
How Supplied
- NDC #62856-799-05, ALOXI Capsules, 0.5 mg (free base), are supplied as light beige opaque soft gelatin capsules, five capsules per bottle, each bottle packaged in a small carton.
Storage
- Store at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [see USP Controlled Room Temperature].
- Protect from light.
Images
Drug Images
{{#ask: Page Name::Palonosetron hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Palonosetron hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Instructions for Patients
- Advise patients of the possibility of serotonin syndrome, especially with concomitant use of ALOXI and another serotonergic agent such as medications to treat depression and migraines. Advise patients to seek immediate medical attention if the following symptoms occur: changes in mental status, autonomic instability, neuromuscular symptoms with or without gastrointestinal symptoms.
- Patients should be instructed to read the Patient Information.
Precautions with Alcohol
- Alcohol-Palonosetron hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ALOXI
Look-Alike Drug Names
There is limited information regarding Palonosetron hydrochloride Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Palonosetron hydrochloride
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Palonosetron hydrochloride |Label Name=Aloxi ingredients and appearance.png
}}
{{#subobject:
|Label Page=Palonosetron hydrochloride |Label Name=Aloxi image.jpg
}}
{{#subobject:
|Label Page=Palonosetron hydrochloride |Label Name=Aloxi patient information.png
}}