Nicotine (nasal)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nicotine (nasal) is a Dependency Agent that is FDA approved for the treatment of smoking cessation for the relief of nicotine withdrawal symptoms. Common adverse reactions include skin irritation, nasal irritation, nasal spray, oral irritation, dizziness, headache, insomnia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Nicotine NS is indicated as an aid to smoking cessation for the relief of nicotine withdrawal symptoms. Nicotine NS therapy should be used as a part of a comprehensive behavioral smoking cessation program.
- The safety and efficacy of the continued use of nicotine NS for periods longer than 6 months have not been adequately studied and such use is not recommended.
Dosage
- It is important that patients understand the instructions for use of nicotine NS, and have their questions answered. They should clearly understand the directions for using nicotine NS and safely disposing of the used container. They should be instructed to stop smoking completely when they begin using the product.
- Patients should be instructed not to sniff, swallow or inhale through the nose as the spray is being administered. They should also be advised to administer the spray with the head tilted back slightly.
- The dose of nicotine NS, should be individualized on the basis of each patient's nicotine dependence and the occurrence of symptoms of nicotine excess .
- Each actuation of nicotine NS delivers a metered 50 microliter spray containing 0.5 mg of nicotine. One dose is 1 mg of nicotine (2 sprays, one in each nostril).
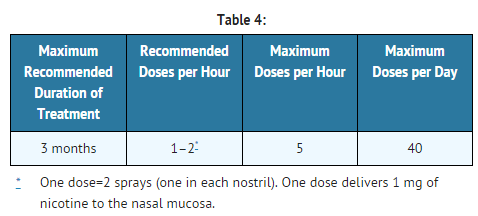
- Patients should be started with 1 or 2 doses per hour, which may be increased up to a maximum recommended dose of 40 mg (80 sprays, somewhat less than 1/2 bottle) per day. For best results, patients should be encouraged to use at least the recommended minimum of 8 doses per day, as less is unlikely to be effective. In clinical trials, the patients who successfully quit smoking used the product heavily when nicotine withdrawal was at its peak, sometimes up to the recommended maximum of 40 doses per day ( in heavier smokers). Dosing recommendations are summarized in Table 4.
- No tapering strategy has been shown to be optimal in clinical studies. Many patients simply stopped using the spray at their last clinic visit.
- Recommended strategies for discontinuation of use include suggesting that patients: use only 1/2 a dose (1 spray) at a time, use the spray less frequently, keep a tally of daily usage, try to meet a steadily reducing usage target, skip a dose by not medicating every hour, or set a planned "quit date" for stopping use of the spray.
Individualization of Dosage
- The success or failure of smoking cessation is influenced by the quality, intensity and frequency of supportive care. Patients are more likely to quit smoking if they are seen frequently and participate in formal smoking cessation programs.
- The goal of nicotine NS therapy is complete abstinence. If a patient is unable to stop smoking by the fourth week of therapy, treatment should probably be discontinued.
- Patients who fail to quit on any attempt may benefit from interventions to improve their chances for success on subsequent attempts. Patients who were unsuccessful should be counseled and should then probably be given a "therapy holiday" before the next attempt. A new quit attempt should be encouraged when conditions are more favorable.
- Based on the clinical trials, a reasonable approach to assisting patients in their attempt to quit smoking is to begin initial treatment, using the recommended dosage. Regular use of the spray during the first week of treatment may help patients adapt to the irritant effects of the spray. Dosage can then be adjusted in those subjects with signs or symptoms of nicotine withdrawal or excess. Patients who are successfully abstinent on nicotine NS should be treated at the selected dosage for up to 8 weeks, following which use of the spray should be discontinued over the next 4 to 6 weeks. Some patients may not require gradual reduction of dosage and may abruptly stop treatment successfully. Treatment with nicotine NS for longer periods has not been shown to improve outcome, and the safety of use for periods longer than 6 months has not been established.
- The symptoms of nicotine withdrawal overlap those of nicotine excess . Since patients using nicotine NS may also smoke intermittently, it is sometimes difficult to determine if patients are experiencing nicotine withdrawal or nicotine excess. Controlled clinical trials of nicotine products suggest that palpitations, nausea and sweating are more often symptoms of nicotine excess, whereas anxiety, nervousness and irritability are more often symptoms of nicotine withdrawal.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nicotine (nasal) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nicotine (nasal) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Nicotine (nasal) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nicotine (nasal) in pediatric patients.
Contraindications
- Use of nicotine NS therapy is contraindicated in patients with known hypersensitivity or allergy to nicotine or to any component of the product.
Warnings
- Nicotine from any source can be toxic and addictive. Smoking causes lung disease, cancer, and heart disease and may adversely affect pregnant women or the fetus. For any smoker, with or without concomitant disease or pregnancy, the risk of nicotine replacement in a smoking cessation program should be weighed against the hazard of continued smoking, and the likelihood of achieving cessation of smoking without nicotine replacement.
- Pregnancy, Warning
- Tobacco smoke, which has been shown to be harmful to the fetus, contains nicotine, hydrogen cyanide, and carbon monoxide. Nicotine has been shown in animal studies to cause fetal harm. It is therefore presumed that nicotine NS can cause fetal harm when administered to a pregnant woman. The effect of nicotine delivery by nicotine NS has not been examined in pregnancy . Therefore, pregnant smokers should be encouraged to attempt cessation using educational and behavioral interventions before using pharmacological approaches. If nicotine NS is used during pregnancy, or if the patient becomes pregnant while using it, the patient should be apprised of the potential hazard to the fetus.
- Safety Note Concerning Children
- The amounts of nicotine that are tolerated by adult smokers can produce symptoms of poisoning and could prove fatal if nicotine NS is used or ingested by children or pets. A full bottle of nicotine NS contains 100 mg of nicotine, some of which will still be in the bottle when it is discarded. Therefore, patients should be cautioned to keep both used and unused containers of nicotine NS out of the reach of children and pets.
Precautions
- The patient should be urged to stop smoking completely when initiating nicotine NS therapy . Patients should be informed that if they continue to smoke while using the product, they may experience adverse effects due to peak nicotine levels higher than those experienced from smoking alone. If there is a clinically significant increase in cardiovascular or other effects attributable to nicotine, the treatment should be discontinued . Physicians should anticipate that concomitant medications may need dosage adjustment
- Sustained use (beyond 6 months) of nicotine NS by patients who stop smoking is not recommended and should be discouraged .
- Use of nicotine NS is not recommended in patients with known chronic nasal disorders (e.g. allergy, rhinitis, nasal polyps and sinusitis) since such use has not been adequately studied.
- Asthma, Bronchospasm and Reactive Airway Disease
- Exacerbation of bronchospasm in patients with pre-existing asthma has been reported. Use of nicotine NS in patients with severe reactive airway disease is not recommended.
- Effect of nicotine NS on the Nasal Mucosa
- Topical application of either nicotine or tobacco products is irritating to the nasal mucosa and physicians should consider both the risks and benefits to the patient before initiating or continuing nicotine NS therapy.
- The effect of nicotine NS on the nasal mucosa was studied in 39 cigarette smokers who used nicotine NS for 1 month. When compared to baseline, random biopsies taken after four weeks of treatment revealed 1 patient with persistence of pre-existing dysplasia and 1 patient with a newly found dysplasia. In both, dysplasia was not seen after a recovery period of eight weeks.
- Forty-two patients who used nicotine NS for more than 6 months underwent follow-up ear, nose and throat examinations 1 to 3 months after discontinuing the use of the spray. Many reported local irritant effects of the spray during spray use, but none showed persistent mucosal injury that the examining physician could attribute to use of the product.
- The clinical significance of these findings is not known, but extended use of the product beyond six months is not recommended.
- Cardiovascular or Peripheral Vascular Diseases
- The risks of nicotine replacement in patients with cardiovascular and peripheral vascular diseases should be weighed against the benefits of including nicotine replacement in a smoking cessation program for them. Specifically, patients with coronary heart disease (history of myocardial infarction and/or angina pectoris), serious cardiac arrhythmias, or vasospastic diseases (Buerger's disease, Prinzmetal's variant angina and Raynaud's phenomena) should be evaluated carefully before nicotine replacement is prescribed.
- Tachycardia occurring in association with nicotine replacement therapy has been reported. No serious cardiovascular events were reported in clinical studies with nicotine NS, but if symptoms occur, its use should be discontinued.
- Nicotine NS generally should not be used in patients during the immediate postmyocardial infarction period, nor in patients with serious arrhythmias, or with severe or worsening angina.
- Renal or Hepatic Insufficiency
- The pharmacokinetics of nicotine have not been studied in the elderly or in patients with renal or hepatic impairment. However, given that nicotine is extensively metabolized and that its total system clearance is dependent on liver blood flow, some influence of hepatic impairment on drug kinetics (reduced clearance) should be anticipated. Only severe renal impairment would be expected to affect the clearance of nicotine or its metabolites from the circulation.
- Endocrine Diseases
- nicotine NS therapy should be used with caution in patients with hyperthyroidism, pheochromocytoma or insulin-dependent diabetes, since nicotine causes the release of catecholamines by the adrenal medulla.
- Peptic Ulcer Disease
- Nicotine delays healing in peptic ulcer disease; therefore, nicotine NS therapy should be used with caution in patients with active peptic ulcers and only when the benefits of including nicotine replacement in a smoking cessation program outweigh the risks.
- Accelerated Hypertension
- Nicotine therapy constitutes a risk factor for development of malignant hypertension in patients with accelerated hypertension; therefore, nicotine NS therapy should be used with caution in these patients and only when the benefits of including nicotine replacement in a smoking cessation program outweigh the risks.
Adverse Reactions
Clinical Trials Experience
- Assessment of adverse events in the 730 patients who participated in controlled clinical trials is complicated by the occurrence of signs and symptoms of nicotine withdrawal in some patients and nicotine excess in others. The incidence of adverse events is confounded by the many minor complaints that smokers commonly have, by continued smoking by many patients and the local irritation from both active drug and the pepper placebo. No serious adverse events were reported during the trials.
- Common Smoker's Complaints
- Common complaints experienced by the smokers in the study (users of both active and placebo spray) include: chest tightness, dyspepsia, paraesthesia (tingling) in limbs, constipation, and stomatitis.
- Tobacco Withdrawal Symptoms
- Symptoms of tobacco withdrawal were frequent in users of both active and placebo sprays. Common withdrawal symptoms seen in over 5% of patients included: anxiety, irritability, restlessness, cravings, dizziness, impaired concentration, weight increase, emotional lability, somnolence and fatigue, increased sweating, and insomnia. Less frequently seen probable withdrawal symptoms (under 5%) included: confusion, depression, apathy, tremor, increased appetite, incoordination and increased dreaming.
- Anxiety, irritability, restlessness and tobacco cravings occurred about equally in both groups, while other symptoms tended to be slightly more common on placebo spray.
- Effects of the Spray
- nicotine NS and the pepper-containing placebo were both associated with irritant side effects on the nasopharyngeal and ocular tissues. During the first 2 days of treatment, nasal irritation was reported by nearly all (94%) of the patients, the majority of whom rated it as either moderate or severe. Both the frequency and severity of nasal irritation declined with continued use of nicotine NS but was still experienced by most (81%) of the patients after 3 weeks of treatment, with most patients rating it as moderate or mild.
- Other common side-effects for both active and placebo groups were: runny nose, throat irritation, watering eyes, sneezing, and coughing.
- The following local events were reported somewhat more commonly for active than for placebo spray: nasal congestion, subjective comments related to the taste or use of the dosage form, sinus irritation, transient epistaxis, eye irritation, transient changes in sense of smell, pharyngitis, paraethesias of the nose, mouth or head, numbness of the nose, or mouth, burning of the nose or eyes, earache, facial flushing, transient changes in sense of taste, hoarseness, nasal ulcer or blister.
- Effects of Nicotine
- Feelings of dependence on the spray were reported by more patients on active spray than placebo. Drug-like effects such as calming were also more frequent on active spray.
- Other Adverse Effects
- Adverse events which could not be classified and listed above and which were reported by >1% of patients on active spray are listed in the following table:
- Adverse Events Not Attributable to Intercurrent Illness
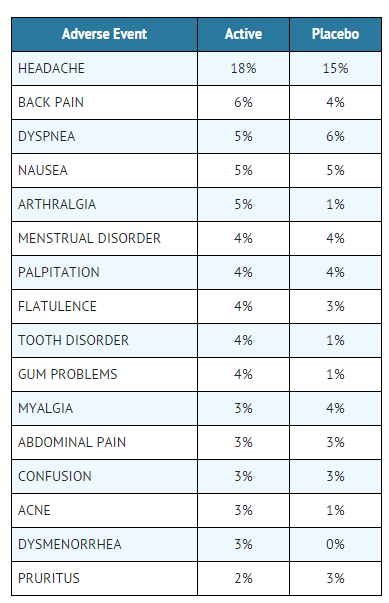
This image is provided by the National Library of Medicine.
- Adverse events reported with a frequency of <1% among active spray users are listed below:
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nicotine (nasal) in the drug label.
Drug Interactions
- The extent of absorption and peak plasma concentration is slightly reduced in patients with the common cold/rhinitis. In addition, the time to peak concentration is prolonged. The use of a nasal vasoconstrictor such as xylometazoline in patients with rhinitis will further prolong the time to peak . Smoking cessation, with or without nicotine replacement, may alter the pharmacokinetics of certain concomitant medications.
Use in Specific Populations
Pregnancy
- Pregnancy Category D
- The harmful effects of cigarette smoking on maternal and fetal health are clearly established. These include low birth weight, an increased risk of spontaneous abortion, and increased perinatal mortality. The specific effects of nicotine NS on fetal development are unknown. Therefore pregnant smokers should be encouraged to attempt cessation using educational and behavioral interventions before using pharmacological approaches.
- Spontaneous abortion during nicotine replacement therapy has been reported; as with smoking, nicotine as a contributing factor cannot be excluded.
- nicotine NS should be used during pregnancy only if the likelihood of smoking cessation justifies the potential risk of using it by the pregnant patient, who might continue to smoke.
- Teratogenicity
- Animal Studies
- Nicotine was shown to produce skeletal abnormalities in the offspring of mice when toxic doses were given to the dams (25 mg/kg IP or SC).
- Human Studies
- Nicotine teratogenicity has not been studied in humans except as a component of cigarette smoke (each cigarette smoked delivers about 1 mg of nicotine). It has not been possible to conclude whether cigarette smoking is teratogenic to humans.
- Other Effects
- Animal Studies
- A nicotine bolus (up to 2 mg/kg) to pregnant rhesus monkeys caused acidosis, hypercarbia, and hypotension (fetal and maternal concentrations were about 20 times those achieved after smoking one cigarette in 5 minutes). Fetal breathing movements were reduced in the fetal lamb after intravenous injection of 0.25 mg/kg nicotine to the ewe (equivalent to smoking 1 cigarette every 20 seconds for 5 minutes). Uterine blood flow was reduced about 30% after infusion of 0.1 µg/kg/min nicotine to pregnant rhesus monkeys (equivalent to smoking about six cigarettes every minute for 20 minutes).
- Human Experience
- Cigarette smoking during pregnancy is associated with an increased risk of spontaneous abortion, low birth weight infants and perinatal mortality. Nicotine and carbon monoxide are considered the most likely mediators of these outcomes. The effects of cigarette smoking on fetal cardiovascular parameters have been studied near term. Cigarettes increased fetal aortic blood flow and heart rate and decreased uterine blood flow and fetal breathing movements. nicotine NS has not been studied in pregnant women.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nicotine (nasal) in women who are pregnant.
Labor and Delivery
- nicotine NS is not recommended for use during labor and delivery. The effect of nicotine on a mother or the fetus during labor is unknown.
Nursing Mothers
- Caution should be exercised when nicotine NS is administered to nursing mothers. The safety of nicotine NS therapy in nursing infants has not been examined. Nicotine passes freely into breast milk; the milk to plasma ratio averages 2.9. Nicotine is absorbed orally. An infant has the ability to clear nicotine by hepatic first-pass clearance; however, the efficiency of removal is probably lowest at birth. Nicotine concentrations in milk can be expected to be lower with nicotine NS when used as recommended than with cigarette smoking, as maternal plasma nicotine concentrations are generally reduced with nicotine replacement. The risk of exposure of the infant to nicotine from nicotine NS therapy should be weighed against the risks associated with the infant's exposure to nicotine from continued smoking by the mother (passive smoke exposure and contamination of breast milk with other components of tobacco smoke) and from nicotine NS alone, or in combination with continued smoking.
Pediatric Use
- nicotine NS therapy is not recommended for use in the pediatric population because its safety and effectiveness in children and adolescents who smoke have not been evaluated.
Geriatic Use
- Clinical studies of nicotine NS did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger subjects. Other reports on clinical experience have not identified differences between older and younger patients. In general, dosage selection for an elderly patient should be cautious, usually starting at the low end of the dosage range reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease.
Gender
There is no FDA guidance on the use of Nicotine (nasal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nicotine (nasal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nicotine (nasal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nicotine (nasal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nicotine (nasal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nicotine (nasal) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Nasal
Monitoring
There is limited information regarding Monitoring of Nicotine (nasal) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Nicotine (nasal) in the drug label.
Overdosage
- The oral LD50 for nicotine is >5 mg/kg in dogs and >24 mg/kg in rodents. Death is due to respiratory paralysis. The oral minimum acute lethal dose for nicotine in adult humans is reported to be 40 to 60 mg (<1 mg/kg). A full bottle of nicotine NS contains 100 mg of nicotine.
- nicotine NS would be expected to be irritating if sprayed in the eyes, mouth or ears. Eye exposure should be treated with copious irrigation with water for 20 minutes. Large oral nicotine ingestions cause vomiting, and the consequences of an overdose will vary; should this occur, patients should contact their physician immediately. For additional emergency information, call your regional poison center.
- Signs and Symptoms of Nicotine Toxicity
- Signs and symptoms of an overdose of nicotine NS would be expected to be the same as those of acute nicotine poisoning including: pallor, cold sweat, nausea, salivation, vomiting, abdominal pain, diarrhea, headache, dizziness, disturbed hearing and vision, tremor, mental confusion, and weakness. Prostration, hypotension, and respiratory failure may ensue with large overdoses. Lethal doses produce convulsions quickly and death follows as a result of peripheral or central respiratory paralysis or, less frequently, cardiac failure.
- Overdose from Ingestion
- If emesis has not occurred, it should be induced in conscious patients with a suitable emetic followed by an appropriate dose of activated charcoal. In unconscious patients with a secure airway, instill activated charcoal via a nasogastric tube. A saline cathartic or sorbitol may be added to the first dose of activated charcoal.
- Management of Nicotine Poisoning
- Other supportive measures include diazepam or barbiturates for seizures, atropine for excessive bronchial secretions or diarrhea, respiratory support for respiratory failure, and vigorous fluid support for hypotension and cardiovascular collapse.
Pharmacology
There is limited information regarding Nicotine (nasal) Pharmacology in the drug label.
Mechanism of Action
- Nicotine, the chief alkaloid in tobacco products, binds stereo-selectively to nicotinic-cholinergic receptors at the autonomic ganglia, in the adrenal medulla, at neuromuscular junctions, and in the brain. Two types of central nervous system effects are believed to be the basis of nicotine's positively reinforcing properties. A stimulating effect is exerted mainly in the cortex via the locus ceruleus and a reward effect is exerted in the limbic system. At low doses, the stimulant effects predominate while at high doses the reward effects predominate. Intermittent intravenous administration of nicotine activates neurohormonal pathways, releasing acetylcholine, norepinephrine, dopamine, serotonin, vasopressin, beta-endorphin, growth hormone, and ACTH.
Structure
- nicotine® NS (nicotine nasal spray) is an aqueous solution of nicotine intended for administration as a metered spray to the nasal mucosa.
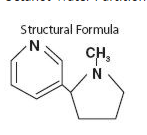
- Nicotine is a tertiary amine composed of pyridine and a pyrrolidine ring. It is a colorless to pale yellow, freely water-soluble, strongly alkaline, oily, volatile, hygroscopic liquid obtained from the tobacco plant. Nicotine has a characteristic pungent odor and turns brown on exposure to air or light. Of its two stereoisomers, S(-)nicotine is the more active. It is the prevalent form in tobacco, and is the form in nicotine NS. The free alkaloid is absorbed rapidly through skin, mucous membranes, and the respiratory tract.
- Chemical Name: S-3-(1-methyl-2-pyrrolidinyl) pyridine
- Molecular Formula: C10H14N2
- Molecular Weight: 162.23
- Ionization Constants: pKa1 = 7.84, pKa2 = 3.04 at 15°C
- Octanol-Water Partition Coefficient: 15:1 at pH 7
- Each 10 mL spray bottle contains 100 mg nicotine (10 mg/mL) in an inactive vehicle containing disodium phosphate, sodium dihydrogen phosphate, citric acid, methylparaben, propylparaben, edetate disodium, sodium chloride, polysorbate 80, aroma and water. The solution is isotonic with a pH of 7. It contains no chlorofluorocarbons.
- After priming the delivery system for nicotine NS, each actuation of the unit delivers a metered dose spray containing approximately 0.5 mg of nicotine. The size of the droplets produced by the unit is in excess of 8 microns. One nicotine NS unit delivers approximately 200 applications.
Pharmacodynamics
- The cardiovascular effects of nicotine include peripheral vasoconstriction, tachycardia, and elevated blood pressure. Acute and chronic tolerance to nicotine develops from smoking tobacco or ingesting nicotine preparations. Acute tolerance (a reduction in response for a given dose) develops rapidly (less than 1 hour), but not at the same rate for different physiologic effects (skin temperature, heart rate, subjective effects). Withdrawal symptoms such as cigarette craving can be reduced in most individuals by plasma nicotine levels lower than those from smoking.
- Withdrawal from nicotine in addicted individuals can be characterized by craving, nervousness, restlessness, irritability, mood lability, anxiety, drowsiness, sleep disturbances, impaired concentration, increased appetite, minor somatic complaints (headache, myalgia, constipation, fatigue), and weight gain. Nicotine toxicity is characterized by nausea, abdominal pain, vomiting, diarrhea, diaphoresis, flushing, dizziness, disturbed hearing and vision, confusion, weakness, palpitations, altered respiration and hypotension.
- Both smoking and nicotine can increase circulating cortisol and catecholamines, and tolerance does not develop to the catecholamine-releasing effects of nicotine. Changes in the response to a concomitantly administered adrenergic agonist or antagonist should be watched for when nicotine intake is altered during nicotine NS therapy and/or smoking cessation
Pharmacokinetics
- Each actuation of nicotine NS delivers a metered 50 microliter spray containing approximately 0.5 mg of nicotine. One dose is considered 1 mg of nicotine (2 sprays, one in each nostril).
- Absorption
- Following administration of 2 sprays of nicotine NS approximately 53% ± 16% (Mean ± SD) enters the systemic circulation. No significant difference in rate or extent of absorption could be seen due to the deposition of nicotine on different parts of the nasal mucosa. Plasma concentrations of nicotine obtained from 1 dose (1 mg nicotine) of nicotine NS rise rapidly, reaching maximum venous concentrations of 2–12 ng/mL in 4–15 minutes. The apparent absorption half-life of nicotine is approximately 3 minutes. There is wide variation among subjects in their plasma nicotine concentrations from the spray. As a result, after a 1 mg dose of spray approximately 20% of the subjects reached peak nicotine concentrations similar to those seen after smoking one cigarette (7–17 ng/mL). Figure 1 below plots the mean and 5th and 95th percentile nicotine concentrations after a 1 mg single dose of the nasal spray (n=30).
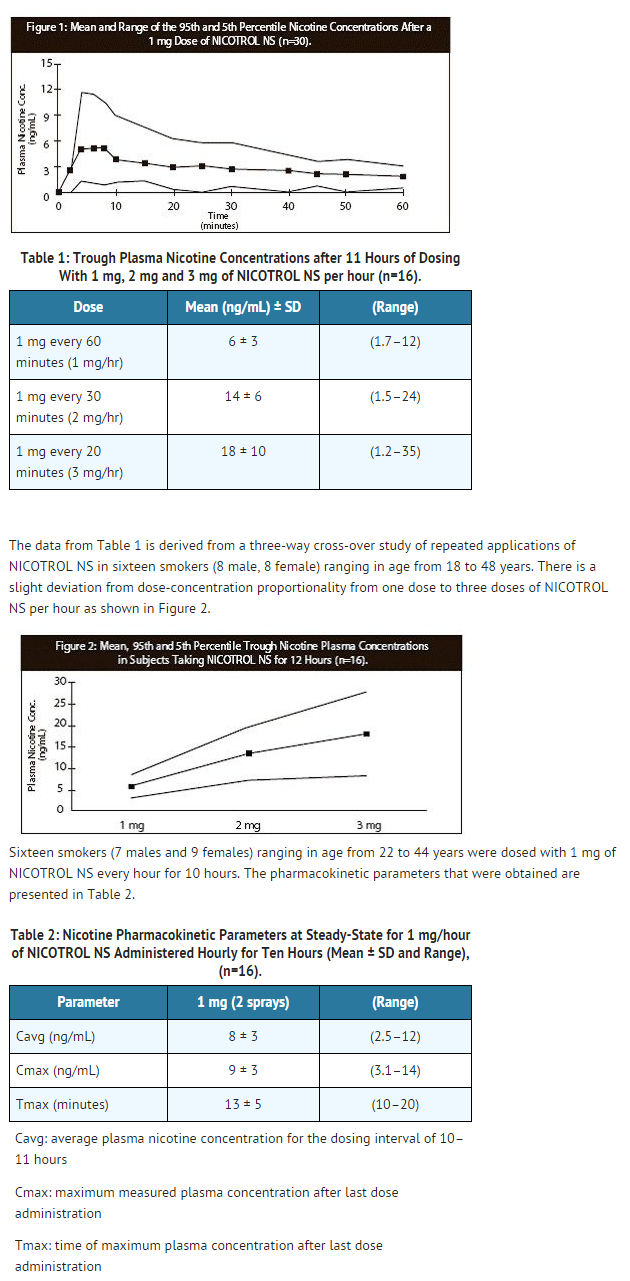
This image is provided by the National Library of Medicine.
Distribution
- The volume of distribution following IV administration of nicotine is approximately 2 to 3 L/kg. Plasma protein binding of nicotine is <5%. Therefore, changes in nicotine binding from use of concomitant drugs or alterations of plasma proteins by disease states would not be expected to have significant effects on nicotine kinetics.
- Metabolism
- More than 20 metabolites of nicotine have been identified, all of which are less active than the parent compound. The primary urinary metabolites are cotinine (15% of the dose) and trans-3-hydroxycotinine (45% of the dose). Cotinine has a half-life of 15 to 20 hours and concentrations that exceed nicotine by 10-fold. The major site for the metabolism of nicotine is the liver. The kidney and lung are also sites of nicotine metabolism.
- Elimination
- About 10% of the nicotine absorbed is excreted unchanged in the urine. This may be increased to up to 30% with high urine flow rates and urinary acidification below pH 5. The average plasma clearance is about 1.2 L/min in a healthy adult smoker. The apparent elimination half-life of nicotine from nicotine NS is 1 to 2 hours.
- Pharmacokinetic Model
- The data were well described by a two-compartment model with first-order input.
- Based on individual fits (N=18) the following parameters were derived after the administration of a 1 mg dose: Absorption rate constant (Ka) = 14.4 ± 7.3 hr-1 (Mean ± SD), Elimination rate constant (Ke) = 0.60 ± 0.53 hr-1, Distribution rate constants (K12) = 4.84 ± 2.57 hr-1, (K21) = 4.35 ± 2.30 hr-1, Volume of distribution over fraction absorbed (V/F) = 2.73 ± 0.82 L/kg in 8 female and 10 male adults weighing 76 ± 15 kg.
- Gender Differences
- Intersubject variability (50% coefficient of variation) among the pharmacokinetic parameters (AUC, Cmax and Clearance/kg) were observed for both genders. There were no differences between females or males in the kinetics of nicotine NS.
- Drug/Drug Interactions
- The extent of absorption is slightly reduced (approximately 10%) in patients with the common cold/rhinitis. In patients with rhinitis the peak plasma concentration is reduced by approximately 20% (concentrations are lower by 1.5 ng/mL on average) and the time to peak concentration prolonged by approximately 30% (delayed by 7 minutes on average). The use of a nasal vasoconstrictor such as xylometazoline in patients with rhinitis will further prolong the time to peak by approximately 40% (delayed by 15 minutes on average), but the peak plasma concentration remains on average the same as those with rhinitis.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment Of Fertility
- Nicotine itself does not appear to be a carcinogen in laboratory animals. However, nicotine and its metabolites increased the incidences of tumors in the cheek pouches of hamsters and forestomach of F344 rats, respectively, when given in combination with tumor-initiators. One study, which could not be replicated, suggested that cotinine, the primary metabolite of nicotine, may cause lymphoreticular sarcoma in the large intestine of rats.
- Neither nicotine norcotinine were mutagenic in the Ames salmonella test. Nicotine induced repairable DNA damage in an E. coli test system. Nicotine was shown to be genotoxic in a test system using Chinese hamster ovary cells. In rats and rabbits, implantation can be delayed or inhibited by a reduction in DNA synthesis that appears to be caused by nicotine. Studies have shown a decrease in litter size in rats treated with nicotine during gestation.
Clinical Studies
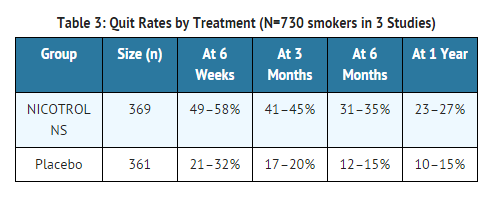
- The efficacy of nicotine NS therapy as an aid to smoking cessation was demonstrated in three single-center, placebo-controlled, double-blind trials with a total of 730 patients. One of the trials used nicotine NS with individual counseling while the other two used group support. Patients with severe or symptomatic cardiovascular disease, hypertension, asthma, diabetes or severe allergy were not included in the studies. The amount of nicotine NS used was left to the discretion of each patient, with a minimum dose of 8 mg/day and a maximum dose of 40 mg/day.
- In all three studies, the recommended duration of treatment was 3 months; however in two of these trials, 241 patients were permitted to continue to use the product for up to 1 year, if they wished. Among the 64 patients abstinent from cigarettes at the end of a year, 23 (36%) were still using the spray, and probable dependence on the spray was seen in several patients (See DRUG ABUSE AND DEPENDENCE).
- Quitting was defined as total abstinence from smoking for at least 4 weeks. The "quit rates" are the percentage of all persons initially enrolled who continuously abstained after week 2 or 4.
- In all three studies, nicotine NS was more effective than placebo at 6 weeks, 3 months, 6 months, and 1 year. The two studies where nicotine NS could be used for more than 6 months did not have a better outcome at 1 year than the study in which nicotine NS was discontinued at 6 months.
- Patients treated with nicotine NS had more relief of the urge to smoke and withdrawal symptoms compared with placebo-treated patients.
- nicotine NS allows the patient to vary the dose of nicotine on a short-term basis. As with other variable dose smoking cessation products, nicotine NS may be useful in the management of highly dependent smokers.
How Supplied
- As with all medicines, especially ones in liquid form, care should be taken in handling nicotine NS during periods of opening and closing the container . If it is dropped it may break. If this occurs, the spill should be cleaned up immediately with an absorbent cloth/paper towel. Care should be taken to avoid contact of the solution with the skin. Broken glass should be picked up carefully, using a broom. The area of the spill should be washed several times. Absorbent material may be disposed of as any other household waste. Should even a small amount of nicotine NS come in contact with the skin, lips, mouth, eyes or ears, the affected area(s) should be immediately rinsed with water only.
- Disposal
- Used bottles of nicotine NS should be disposed of with their child-resistant caps in place. Used bottles should be disposed of in such a way as to prevent access by children or pets. See patient information for further information on handling and disposal.
- How Supplied
- nicotine NS (nicotine nasal spray) 10 mg/mL, is supplied as four 10 mL bottles (NDC 0009-5401-01). Each unit consists of a glass container, mounted with a metered spray pump.
A patient information leaflet is enclosed with the package.
- Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Storage
There is limited information regarding Nicotine (nasal) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nicotine (nasal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nicotine (nasal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- A patient instruction sheet is included in the package of nicotine NS dispensed to the patient. Patients should be encouraged to read the instruction sheet carefully and to ask their physician and pharmacist about the proper use of the product.
- It should be explained to patients that they are likely to experience nasal irritation, which may become less bothersome with continued use.
- Patients must be advised to keep both used and unused containers out of the reach of children and pets.
Precautions with Alcohol
- Alcohol-Nicotine (nasal) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Nicotine (nasal) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Nicotine (nasal) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Nicotine (nasal) |Label Name=Nicotine (nasal)05.png
}}
{{#subobject:
|Label Page=Nicotine (nasal) |Label Name=Nicotine (nasal)06.png
}}
{{#subobject:
|Label Page=Nicotine (nasal) |Label Name=Nicotine (nasal)07.png
}}