Methsuximide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Methsuximide is an anticonvulsant that is FDA approved for the treatment of absence seizures that are refractory to other drugs. Common adverse reactions include weight loss, abdominal pain, constipation, diarrhea, epigastric pain,loss of appetite, nausea, vomiting ataxia, dizziness and somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Methsuximide is indicated for the control of absence seizures (petit mal) that are refractory to other drugs.
Dosage
- Optimum dosage of methsuximide must be determined by trial. A suggested dosage schedule is 300 mg per day for the first week. If required, dosage may be increased thereafter at weekly intervals by 300 mg per day for the three weeks following to a daily dosage of 1.2 g. Because therapeutic effect and tolerance vary among patients, therapy with methsuximide must be individualized according to the response of each patient. Optimal dosage is that amount of methsuximide which is barely sufficient to control seizures so that side effects may be kept to a minimum.
- Methsuximide may be administered in combination with other anticonvulsants when other forms of epilepsy coexist with absence (petit mal).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Methsuximide in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Methsuximide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There is limited information regarding FDA-labeled indications and dosage information of Methsuximide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Methsuximide in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Methsuximide in pediatric patients.
Contraindications
- Methsuximide should not be used in patients with a history of hypersensitivity to succinimides.
Warnings
Blood dyscrasias
- Blood dyscrasias, including some with fatal outcome, have been reported to be associated with the use of succinimides; therefore, periodic blood counts should be performed. Should signs and/or symptoms of infection (eg, sore throat, fever) develop, blood counts should be considered at that point.
Effects on Liver
- It has been reported that succinimides have produced morphological and functional changes in animal liver. For this reason, methsuximide should be administered with extreme caution to patients with known liver or renal disease. Periodic urinalysis and liver function studies are advised for all patients receiving the drug.
Systemic Lupus Erythematosus
- Cases of systemic lupus erythematosus have been reported with the use of succinimides. The physician should be alert to this possibility.
Suicidal Behavior and Ideation
- Antiepileptic drugs (AEDs), including methsuximide, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
- Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
- The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
- The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5–100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs
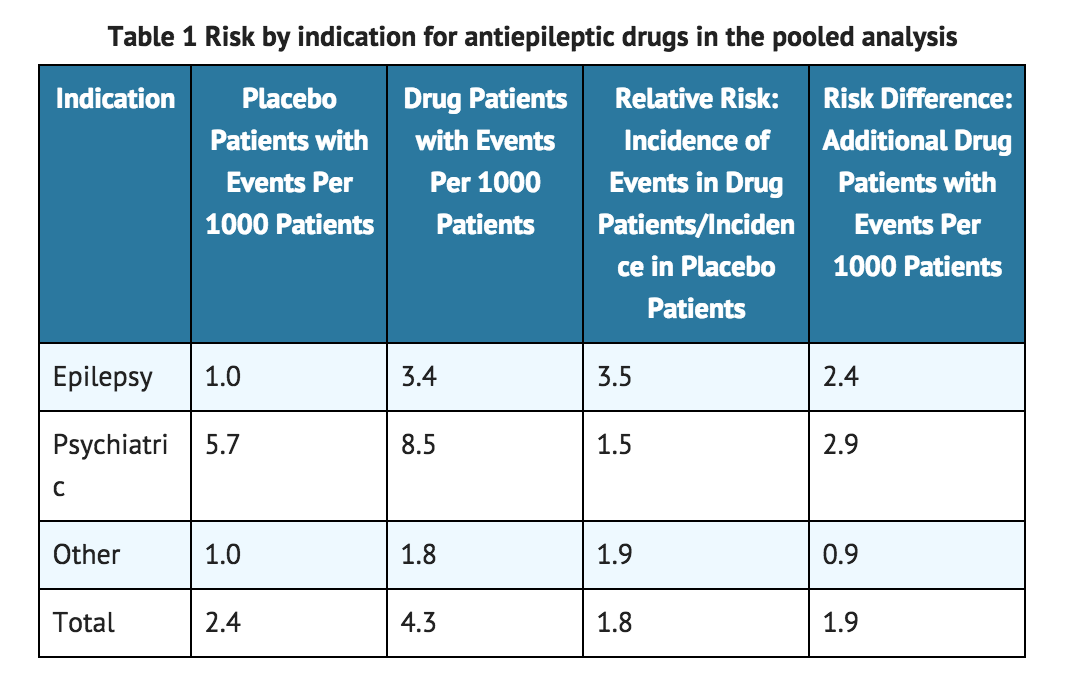
- The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
- Anyone considering prescribing methsuximide or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
- Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Precautions
General
- It is recommended that the physician withdraw the drug slowly on the appearance of unusual depression, aggressiveness, or other behavioral alterations.
- As with other anticonvulsants, it is important to proceed slowly when increasing or decreasing dosage, as well as when adding or eliminating other medication. Abrupt withdrawal of anticonvulsant medication may precipitate absence (petit mal) status.
- Methsuximide, when used alone in mixed types of epilepsy, may increase the frequency of grand mal seizures in some patients.
Information for Patients
- Inform patients of the availability of a Medication Guide, and instruct them to read the Medication Guide prior to taking methsuximide. Instruct patients to take methsuximide only as prescribed.
- Methsuximide may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a motor vehicle or other such activity requiring alertness; therefore, the patient should be cautioned accordingly. Patients taking methsuximide should be advised of the importance of adhering strictly to the prescribed dosage regimen.
- Patients should be instructed to promptly contact their physician if they develop signs and/or symptoms suggesting an infection (eg, sore throat, fever).
- ADVICE TO THE PHARMACIST AND PATIENT: Since methsuximide has a relatively low melting temperature (124° F), storage conditions which may promote high temperatures (closed cars, delivery vans, or storage near steam pipes) should be avoided. Do not dispense or use capsules that are not full or in which contents have melted. Effectiveness may be reduced. Protect from excessive heat (104° F).
- Patients, their caregivers, and families should be counseled that AEDs, including methsuximide, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
- Patients should be encouraged to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334.
Adverse Reactions
Clinical Trials Experience
- Gastrointestinal System: Gastrointestinal symptoms occur frequently and have included nausea or vomiting, anorexia, diarrhea, weight loss, epigastric and abdominal pain, and constipation.
- Hemopoietic System: Hemopoietic complications associated with the administration of methsuximide have included eosinophilia, leukopenia, monocytosis, and pancytopenia with or without bone marrow suppression.
- Nervous System: Neurologic and sensory reactions reported during therapy with methsuximide have included drowsiness, ataxia or dizziness, irritability and nervousness, headache, blurred vision, photophobia, hiccups, and insomnia. Drowsiness, ataxia, and dizziness have been the most frequent side effects noted. Psychologic abnormalities have included confusion, instability, mental slowness, depression, hypochondriacal behavior, and aggressiveness. There have been rare reports of psychosis, suicidal behavior, and auditory hallucinations.
- Integumentary System: Dermatologic manifestations which have occurred with the administration of methsuximide have included urticaria, Stevens-Johnson syndrome, and pruritic erythematous rashes.
- Cardiovascular: Hyperemia.
- Genitourinary System: Proteinuria, microscopic hematuria.
- Body as a Whole: Periorbital edema.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience.
Drug Interactions
- Since methsuximide may interact with concurrently administered antiepileptic drugs, periodic serum level determinations of these drugs may be necessary (eg, methsuximide may increase the plasma concentrations of phenytoin and phenobarbital).
Use in Specific Populations
Pregnancy
- Reports suggest an association between the use of anticonvulsant drugs by women with epilepsy and an elevated incidence of birth defects in children born to these women. Data are more extensive with respect to phenytoin and phenobarbital, but these are also the most commonly prescribed anticonvulsants; less systematic or anecdotal reports suggest a possible similar association with the use of all known anticonvulsant drugs.
- The reports suggesting an elevated incidence of birth defects in children of drug-treated epileptic women cannot be regarded as adequate to prove a definite cause and effect relationship. There are intrinsic methodologic problems in obtaining adequate data on drug teratogenicity in humans; the possibility also exists that other factors, eg, genetic factors or the epileptic condition itself, may be more important than drug therapy in leading to birth defects. The great majority of mothers on anticonvulsant medication deliver normal infants. It is important to note that anticonvulsant drugs should not be discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that the removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy, although it cannot be said with any confidence that even minor seizures do not pose some hazard to the developing embryo or fetus.
- The prescribing physician will wish to weigh these considerations in treating or counseling epileptic women of childbearing potential.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Methsuximide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Methsuximide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Methsuximide in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Methsuximide in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Methsuximide in geriatric settings.
Gender
There is no FDA guidance on the use of Methsuximide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Methsuximide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Methsuximide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Methsuximide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Methsuximide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Methsuximide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral.
Monitoring
- There is limited information regarding drug monitoring.
IV Compatibility
- There is limited information regarding IV Compatibility.
Overdosage
- Acute overdoses may produce nausea, vomiting, and CNS depression including coma with respiratory depression. Methsuximide poisoning may follow a biphasic course. Following an initial comatose state, patients have awakened and then relapsed into a coma within 24 hours. It is believed that an active metabolite of methsuximide, N-desmethylmethsuximide, is responsible for this biphasic profile. It is important to follow plasma levels of N-desmethylmethsuximide in methsuximide poisonings. Levels greater than 40 µg/mL have caused toxicity, and coma has been seen at levels of 150 µg/mL.
Treatment
- Treatment should include emesis (unless the patient is or could rapidly become obtunded, comatose, or convulsing) or gastric lavage, activated charcoal, cathartics, and general supportive measures. Charcoal hemoperfusion may be useful in removing the N-desmethyl metabolite of methsuximide. Forced diuresis and exchange transfusions are ineffective.
Pharmacology
| |
1 : 1 mixture (racemate)Mesuximide
| |
| Systematic (IUPAC) name | |
| (RS)-1,3-dimethyl-3-phenyl-pyrrolidine-2,5-dione | |
| Identifiers | |
| CAS number | |
| ATC code | N03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 203.237 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic (demethylation and glucuronidation) |
| Half life | 1.4–2.6 hours (mesuximide) 28–38 hours (active metabolite) |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- Methsuximide suppresses the paroxysmal three cycle per second spike and wave activity associated with lapses of consciousness which is common in absence seizures (petit mal). The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the central nervous system to convulsive stimuli.
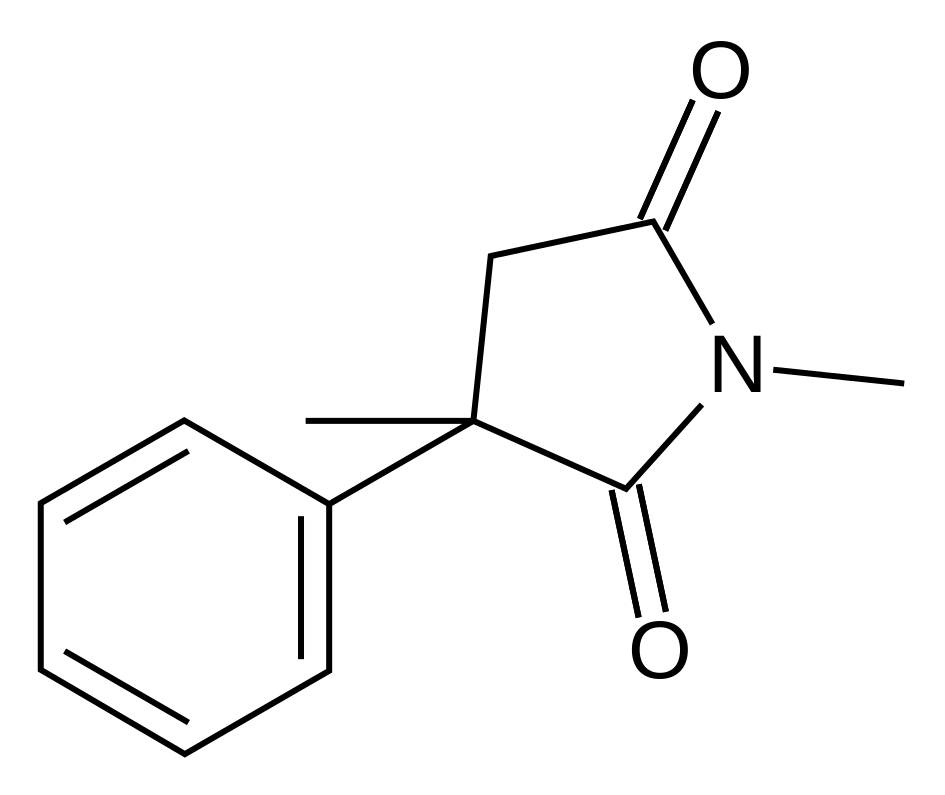
Structure
- Methsuximide is an anticonvulsant succinimide, chemically designated as N,2-Dimethyl-2-phenylsuccinimide, with the following structural formula:

- Each Celontin capsule contains 300 mg methsuximide, USP. Also contains starch, NF. The capsule contains colloidal silicon dioxide, NF; D&C yellow No. 10; FD&C yellow No. 6; gelatin, NF; and sodium lauryl sulfate, NF.
Pharmacodynamics
- There is limited information regarding pharmacodynamics.
Pharmacokinetics
- There is limited information regarding pharmacokinetics.
Nonclinical Toxicology
- There is limited information regarding nonclinical toxicology.
Clinical Studies
- There is limited information regarding Clinical Studies.
How Supplied
- N 0071-0525-24 (P-D 525)–Celontin Capsules, #1 capsule each containing 300 mg methsuximide; bottles of 100.
Storage
- Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature].
- Protect from light and moisture. Protect from excessive heat 40°C (104°F).
Images
Drug Images
{{#ask: Page Name::Methsuximide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Pfizer
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0071-0525-24
Celontin® (Methsuximide Capsules, USP) 300 mg 100 Capsules


{{#ask: Label Page::Methsuximide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Methsuximide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CELONTIN ®[1]
Look-Alike Drug Names
There is limited information regarding Methsuximide Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.