Medazepam
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Elimination half-life | 36-150 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H15ClN2 |
| Molar mass | 270.8 |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Medazepam |
|
Articles |
|---|
|
Most recent articles on Medazepam |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Medazepam at Clinical Trials.gov Clinical Trials on Medazepam at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Medazepam
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Medazepam Discussion groups on Medazepam Directions to Hospitals Treating Medazepam Risk calculators and risk factors for Medazepam
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Medazepam |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Medazepam is a drug that is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative, and skeletal muscle relaxant properties. It is known by the following brand names: Azepamid, Nobrium, Tranquirax (mixed with Bevonium), Rudotel, Raporan, Ansilan and Mezapam.[1] Medazepam is a long-acting benzodiazepine drug. The half-life of medazepam is 36 – 200 hours.[2]
Pharmacology
Benzodiazepine drugs including medazepam increase the inhibitory processes in the cerebral cortex by allosteric modulation of the GABA receptor.[3] Benzodiazepines may also act via micromolar benzodiazepine-binding sites as Ca2+ channel blockers and significantly inhibited depolarization-sensitive calcium uptake in experiments with cell components from rat brains. This has been conjectured as a mechanism for high dose effects against seizures in a study.[4] It has major active benzodiazepine metabolites, which gives it a more prolonged therapeutic effects after administration.[5]
Chemistry
Medazepam can be synthesized in various ways. One is via the reduction of the carbonyl group in diazepam (lacking methyl in Ex 1) by lithium aluminium hydride. N.B. If diazepam is reduced with LAH as in Ex 9, actually the product produced is 7-chloro-1-methyl-5-phenyl-1,2,4,5-tetrahydro-3H-1,4-benzodiazepine, not medazepam.

A second way of making medazepam consists of the initial reduction of the carbonyl group by lithium aluminum hydride into 7-chloro-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one—the first intermediate product in the synthesis of diazepam—which is synthesized by the cyclocondensation of 2-amino-5-chlorobenzophenone with glycine ethyl ester into 7-chloro-2,3-dihydro-5-phenyl-1H-1,4-benzodiazepine, and the subsequent methylation of the secondary amine nitrogen atom of the resulting product by methyl iodide, using sodium hydride as a base.

A third method of making medazepam consists of a new way of making 7-chloro-2,3-dihydro-5-phenyl-1H-1,4-benzodiazepine, which consists in heterocyclization of 1-(2,5-dichlorophenyl)-1-phenylimine with ethylenediamine. The starting 1-(2,5-dichlorophenyl)-1-phenylimine is synthesized by the reaction of 2,5-dichlorobenzonitrile with phenylmagnesium bromide.

A fourth method of making medazepam from 4-chloro-N-methylaniline is suggested. The last is reacted with aziridine (or ethylene imine) in the presence of aluminum chloride, giving N-(4-chlorophenyl)-N-methylethylenediamine.[7] Acylation of the resulting product with BzCl gives the respective amide, which cyclizes into the desired medazepam using phosphorus oxychloride.
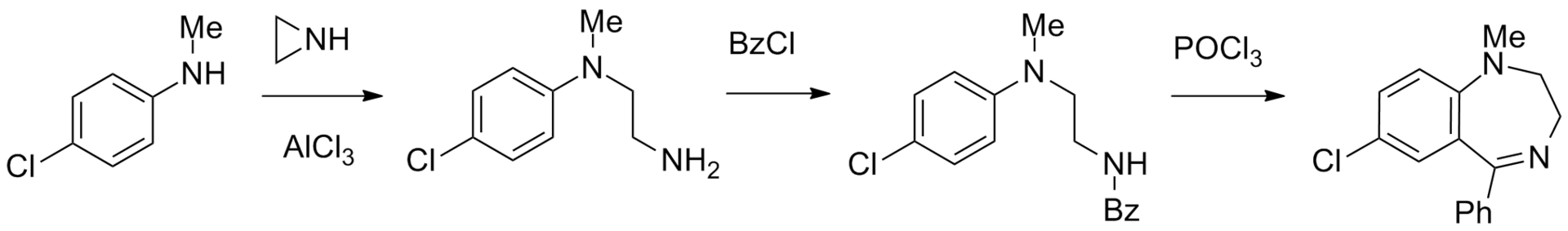
A fifth method also exists:

See also
References
External links
Template:Benzodiazepines Template:Anxiolytics
- ↑ Encyclopedia of Drugs: Benzodiazepines
- ↑ Professor heather Ashton (April 2007). "BENZODIAZEPINE EQUIVALENCY TABLE". Retrieved September 23, 2007.
- ↑ Zakusov VV; Ostrovskaya RU; Kozhechkin SN; Markovich VV; Molodavkin GM; Voronina TA. (October 1977). "Further evidence for GABA-ergic mechanisms in the action of benzodiazepines". Archives internationales de pharmacodynamie et de thérapie. 229 (2): 313–26. PMID 23084.
- ↑ Taft WC; DeLorenzo RJ (May 1984). "Micromolar-affinity benzodiazepine receptors regulate voltage-sensitive calcium channels in nerve terminal preparations" (PDF). Proc Natl Acad Sci USA (PDF). 81 (10): 3118–22. doi:10.1073/pnas.81.10.3118. PMC 345232. PMID 6328498.
- ↑ Jochemsen R, Breimer DD (1984). "Pharmacokinetics of benzodiazepines: metabolic pathways and plasma level profiles". Curr Med Res Opin. 8 Suppl 4: 60–79. doi:10.1185/03007998409109545. PMID 6144464.
- ↑ Template:Cite doi
- ↑ GB 1153103
- ↑ Template:Cite doi
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed CASNo identifier
- Articles with changed DrugBank identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drug
- Benzodiazepines
- Organochlorides