Lambert-Eaton myasthenic syndrome
| Lambert-Eaton myasthenic syndrome | |
 | |
|---|---|
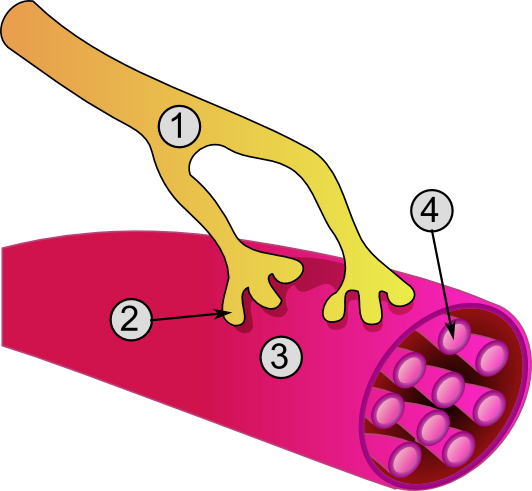
| Global view of a neuromuscular junction: 1. Axon 2. Motor end-plate 3. Muscle fiber 4. Myofibril | |
| ICD-10 | G73.1 |
| ICD-9 | 358.1 |
| DiseasesDB | 4030 |
| MedlinePlus | 000710 |
| MeSH | D015624 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2] Indhumathi Balaraman M.B.B.S[[3]]
Synonyms and keywords: Eaton-Lambert syndrome; Lambert-Eaton syndrome; LEMS
Overview
Lambert-Eaton myasthenic syndrome (LEMS) is a rare autoimmune disorder which affects the nerve-muscle (neuromuscular) junction.
The disease is usually observed in middle aged and older people but children and young people can be affected, as well. Due to the infrequency of the condition, the exact incidence is unknown.
Historical Perspective
- Anderson was the first person to mention a case with possible clinical findings of LEMS in 1953, but Lambert, Eaton and Rooke were the first physicians to substantially describe the clinical and electrophysiological findings of the disease in 1966.[1][2]
- Auto-immune self antibodies to the pre-synaptic voltage gated calcium channels leads to neuromuscular block.
Classification
Lambert Eaton syndrome may be classified as : [[4]]
- Paraneoplastic: Associated with malginancies like Lung cancer - Small cell Lung cancer more than non small cell lung cancer or mixed cell carcinomas, prostate cancer, Lymphoproliferative disorder and Thymomas.
- Non-paraneoplastic or Non tumor Lambert Eaton Syndrome: 65% association with HLA-B8-DR3 Haplotype Gene and idiopathic orgin.
PATHOPHYSIOLOGY
Understanding the concept of Neuromuscular junction,Presynaptic Acetylecholine (ACh) concentration,end plate potential, post-synpatic ACh receptors, role of Calcium and Voltage gated Calcium channel(VGCC) and role of potassium is essential to understand the pathophysiology of Lambert Eaton Myasthenic syndrome.
Briefly, The disease is of autoimmune origin, that is, it is caused by antibodies that are directed against the antigens of the neuromuscular junction. In 1989, the previously anticipated antibodies were demonstrated to be directed against presynaptic calcium channels, which are located in neuromuscular junction (see synapse) and are responsible for the efficient release of acetylcholine
In cases with both LEMS and lung cancer (usually small cell type), the antibodies are suggested to be aimed at cancer cells and to bind and affect the antigens in neuromuscular junction accidentally.
===Voltage Gated Calcium Channel=== [[5]]
- Potassium channels and the concentration of potassium ions does not contribute to the ACh release.
The mechanism by which the ACh is released is by increasing the concentration of calcium ions and their influx into the nerve terminal viz Voltage gated calcium channel (VGCC)
VGCC is a transmembrane protein with many subunits that is the main target in LEMS.
The commonly associated subtypes are L-type,N-type,P/Q-Type of alpha-1 subunit of VGCC, of which the P/Q subtype is the most important target in the disorder. N-Type is usually associated with the paraneoplastic LEMS.
VGCC is responsible for releasing the accumulated ACh in nerve terminal. Thereafter ACh binds to its receptor that depolarizes the muscle fibre end plate leading to a muscle contraction by generating a action potential. IgG antibodies of LEMS acts on the VGCC leading to the disorder of the above mechanism resulting in muscle weakness.
In paraneoplastic LEMS, Small cell lung cancer expresses the VGCC on the sufrace of the tumor cells that acts as the antigen to trigger the antibody production causing profound clinical symptoms.No genetic predisposition seen.
In Non-paraneoplastic syndrome,the autoimmunity against the P/Q type VGCC are seen usually in HLA-B8(HLA-Class I) and HLA- DR3 & HLA- DQ2 (HLA -class II) genes.
There are also some patients who do not carry these antibodies in their serum samples and the exact cause of disease in these cases still remains to be determined.
Causes
LEMS is usually a solitary diagnosis but lung cancer (small-cell lung cancer) may accompany the disease in some cases. It may also be associated with cancers such as lymphoma, non-Hodgkin's lymphoma, T-cell leukemia, non-small cell lung cancer, prostate cancer, and thymoma.
Differentiating Lambert-Eaton myasthenic syndrome from other Diseases
Both the etiology and the clinical findings of Lambert-Eaton myasthenic syndrome may resemble myasthenia gravis, but there are many substantial differences between clinical presentations and pathogenetic features of two disorders. In patients with affected ocular and respiratory muscles, the involvement is not as severe as myasthenia gravis.[3][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18]
| Diseases | History and Physical | Diagnostic tests | Other Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Motor Deficit | Sensory deficit | Cranial nerve Involvement | Autonomic dysfunction | Proximal/Distal/Generalized | Ascending/Descending/Systemic | Unilateral (UL)
or Bilateral (BL) or No Lateralization (NL) |
Onset | Lab or Imaging Findings | Specific test | ||
| Adult Botulism | + | - | + | + | Generalized | Descending | BL | Sudden | Toxin test | Blood, Wound, or Stool culture | Diplopia, Hyporeflexia, Hypotonia, possible respiratory paralysis |
| Infant Botulism | + | - | + | + | Generalized | Descending | BL | Sudden | Toxin test | Blood, Wound, or Stool culture | Flaccid paralysis (Floppy baby syndrome), possible respiratory paralysis |
| Guillian-Barre syndrome[19] | + | - | - | - | Generalized | Ascending | BL | Insidious | CSF: ↑Protein
↓Cells |
Clinical & Lumbar Puncture | Progressive ascending paralysis following infection, possible respiratory paralysis |
| Eaton Lambert syndrome[20] | + | - | + | + | Generalized | Systemic | BL | Intermittent | EMG, repetitive nerve stimulation test (RNS) | Voltage gated calcium channel (VGCC) antibody | Diplopia, ptosis, improves with movement (as the day progresses) |
| Myasthenia gravis[21] | + | - | + | + | Generalized | Systemic | BL | Intermittent | EMG, Edrophonium test | Ach receptor antibody | Diplopia, ptosis, worsening with movement (as the day progresses) |
| Electrolyte disturbance[22] | + | + | - | - | Generalized | Systemic | BL | Insidious | Electrolyte panel | ↓Ca++, ↓Mg++, ↓K+ | Possible arrhythmia |
| Organophosphate toxicity[23] | + | + | - | + | Generalized | Ascending | BL | Sudden | Clinical diagnosis: physical exam & history | Clinical suspicion confirmed with RBC AchE activity | History of exposure to insecticide or living in farming environment. with : Diarrhea, Urination, Miosis, Bradycardia, Lacrimation, Emesis, Salivation, Sweating |
| Tick paralysis (Dermacentor tick)[24] | + | - | - | - | Generalized | Ascending | BL | Insidious | Clinical diagnosis: physical exam & history | - | History of outdoor activity in Northeastern United States. The tick is often still latched to the patient at presentation (often in head and neck area) |
| Tetrodotoxin poisoning[25] | + | - | + | + | Generalized | Systemic | BL | Sudden | Clinical diagnosis: physical exam & dietary history | - | History of consumption of puffer fish species. |
| Stroke[26] | +/- | +/- | +/- | +/- | Generalized | Systemic | UL | Sudden | MRI +ve for ischemia or hemorrhage | MRI | Sudden unilateral motor and sensory deficit in a patient with a history of atherosclerotic risk factors (diabetes, hypertension, smoking) or atrial fibrillation. |
| Poliomyelitis[27] | + | + | + | +/- | Proximal > Distal | Systemic | BL or UL | Sudden | PCR of CSF | Asymmetric paralysis following a flu-like syndrome. | |
| Transverse myelitis[28] | + | + | + | + | Proximal > Distal | Systemic | BL or UL | Sudden | MRI & Lumbar puncture | MRI | History of chronic viral or autoimmune disease (e.g. HIV) |
| Neurosyphilis[29][18] | + | + | - | +/- | Generalized | Systemic | BL | Insidious | MRI & Lumbar puncture | CSF VDRL-specifc | History of unprotected sex or multiple sexual partners.
History of genital ulcer (chancre), diffuse maculopapular rash. |
| Muscular dystrophy[31] | + | - | - | - | Proximal > Distal | Systemic | BL | Insidious | Genetic testing | Muscle biopsy | Progressive proximal lower limb weakness with calf pseudohypertrophy in early childhood. Gower sign positive. |
| Multiple sclerosis exacerbation[32] | + | + | + | + | Generalized | Systemic | NL | Sudden | ↑CSF IgG levels
(monoclonal) |
Clinical assessment and MRI [33] | Blurry vision, urinary incontinence, fatigue |
| Amyotrophic lateral sclerosis[34] | + | - | - | - | Generalized | Systemic | BL | Insidious | Normal LP (to rule out DDx) | MRI & LP | Patient initially presents with upper motor neuron deficit (spasticity) followed by lower motor neuron deficit (flaccidity). |
| Inflammatory myopathy[35] | + | - | - | - | Proximal > Distal | Systemic | UL or BL | Insidious | Elevated CK & Aldolase | Muscle biopsy | Progressive proximal muscle weakness in 3rd to 5th decade of life. With or without skin manifestations. |
Epidemiology and Demographics
'''PARANEOPLASTTIC (LEMS)'''[[6]]
- Age of presentation is found to be around 58 years.
- More common in male than female. ( May be explained by the association with the small cell lung cancer )
- Lambert Eaton Myasthenic Syndrome(LEMS) is a rare disorder which has a very low prevalence than Myasthenia Gravis(MG). It is about 40 times less prevalent than MG, perhaps the incidence is only 10 times lesser than the MG per year.
- Association with the malignancy and its lower prevalence may explain the poor prognosis of the disorder.
'''NON-PARANEOPLASTIC (LEMS)'''[[7]]
- Two peaks of incidence in ages around 35 years and a much larger peak at about 60 years.
- Female more than male like other auto immune diseases.
- good prognosis and survival rate is near normal.
Risk Factors
- Smoking may be considered as a risk factor.[[8]]
Natural History, Complications and Prognosis
Possible complications include:
- Difficulty breathing, including respiratory failure
- Difficulty swallowing
- Infections, such as pneumonia
- Injuries from falls and problems with coordination
The symptoms of Lambert-Eaton syndrome may improve by treating the underlying disease, suppressing the immune system, or removing the antibodies. However, not everyone responds well to treatment.
Symptoms
Symptoms may include:
- Weakness or loss of movement that can be more or less severe, including:
- Difficulty chewing
- Difficulty climbing stairs
- Difficulty lifting objects
- Difficulty talking
- Drooping head
- Need to use hands to get up from sitting or lying positions
- Swallowing difficulty, gagging, or choking
- Vision changes such as:
- Blurry vision
- Double vision
- Problems keeping a steady gaze
Symptoms related to the autonomic nervous system usually occur, and include:
- Blood pressure changes
- Dizziness upon standing
- Dry mouth
Diagnosis
Red flag to investigate may be proximal muscle weakness with areflexia and autonomic dysfunction.
SEROLOGY :
- Antibodies against P/Q Voltage gated Calcium Channel(not a definitive test as it may be present in other autoimmune disease and neurological conditions) more frequently than antibodies against N type Calcium channels.
- LEMS with SCLC are found to have Antibodies against SOX1(immunogenic tumor antigen) - 95% specific.
ELECTRODIAGNOSITC TESTING:
- Repetitive Nerve Stimulation (RNS) testing and the Compound muscle action potential amplitude. ( much commonly used and the availability is more common than SFEMG)
Low rate of repetitive nerve stimulation - decrease in the amplitude and clinical response. High rate - increase in clinical response and amplitude recorded. Following exercise or RNS in high frequency shows a cent percent increase in amplitude and response is noted. perhaps to diagnose LEMS percentage equal to or higher than 60% increase in amplitude is required.
- Needle EMG: Action potentials that are unstable.
- Single Fiber Electromyography (SFEMG) has a better diagnostic power than RNS. On increased frequency of firing, the characteristic jitters and the blockade improves.
Physical Examination
Laboratory Findings
- Antibodies to calcium channels
- Incremental response in repetitive nerve stimulation - incremental response is an increased response of muscle fibers to very high frequencies of electrical stimulation. Observed increase in the response of muscle fibers proves that there is a difficulty with the release of acetylcholine and this difficulty can be overwhelmed by intensive stimulation.
Imaging Findings
- There are no [imaging study] findings associated with [disease name].
- [Imaging study 1] is the imaging modality of choice for [disease name].
- On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3].
- [Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3].
Chest X-ray
- Chest x-ray for a possible lung malignancy
Other Diagnostic Studies
- [Disease name] may also be diagnosed using [diagnostic study name].
- Findings on [diagnostic study name] include [finding 1], [finding 2], and [finding 3].
Treatment
Treatment
The main goals of treatment are to:
- Identify and treat any underlying disorders, such as lung cancer
- Give treatment to help with the weakness
Corticosteroids, azathioprine and 3,4-diaminopyridine are used in treatment of LEMS with limited success. In some cases with a progressive and intractable course, plasma exchange or intravenous immunoglobulin can be tried.
A treatment called plasma exchange usually improves symptoms. Plasma exchange involves removing blood plasma from the body and replacing it with donated plasma. This helps to make sure that any harmful proteins (antibodies) that are interfering with nerve function are removed from the body.
Plasmapheresis may also be effective. During this treatment, the blood is removed from the body. The plasma is separated, the antibodies are removed, and the plasma is returned to the body.
Medications that suppress the immune response, such as prednisone, may improve symptoms in some cases. Medications may also include:
- Anticholinesterase medications such as neostigmine or pyridostigmine (although these are not very effective when given alone)
- 3,4 diaminopyridine works by blocking K+ channel efflux in nerve terminal so that action potential duration is increased. Ca2+ channels can then be open for longer time and allow greater acetylcholine release to stimulate muscle at end plate.
Medical Therapy
- There is no treatment for [disease name]; the mainstay of therapy is supportive care.
- The mainstay of therapy for [disease name] is [medical therapy 1] and [medical therapy 2].
- [Medical therapy 1] acts by [mechanism of action 1].
- Response to [medical therapy 1] can be monitored with [test/physical finding/imaging] every [frequency/duration].
Surgery
- Surgery is the mainstay of therapy for [disease name].
- [Surgical procedure] in conjunction with [chemotherapy/radiation] is the most common approach to the treatment of [disease name].
- [Surgical procedure] can only be performed for patients with [disease stage] [disease name].
Prevention
- There are no primary preventive measures available for [disease name].
- Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3].
- Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3].
References
- ↑ Template:WhoNamedIt
- ↑ E. H. Lambert, L. M. Eaton, E. D. Rooke. Defect of neuromuscular conduction associated with malignant neoplasms. American Journal of Physiology, Bethesda, Maryland, 1956, 187: 612-613.
- ↑ 3.0 3.1 Kira R (February 2018). "[Acute Flaccid Myelitis]". Brain Nerve (in Japanese). 70 (2): 99–112. doi:10.11477/mf.1416200962. PMID 29433111.
- ↑ Hopkins SE (November 2017). "Acute Flaccid Myelitis: Etiologic Challenges, Diagnostic and Management Considerations". Curr Treat Options Neurol. 19 (12): 48. doi:10.1007/s11940-017-0480-3. PMID 29181601.
- ↑ Messacar K, Schreiner TL, Van Haren K, Yang M, Glaser CA, Tyler KL, Dominguez SR (September 2016). "Acute flaccid myelitis: A clinical review of US cases 2012-2015". Ann. Neurol. 80 (3): 326–38. doi:10.1002/ana.24730. PMC 5098271. PMID 27422805.
- ↑ Chong PF, Kira R, Mori H, Okumura A, Torisu H, Yasumoto S, Shimizu H, Fujimoto T, Hanaoka N, Kusunoki S, Takahashi T, Oishi K, Tanaka-Taya K (February 2018). "Clinical Features of Acute Flaccid Myelitis Temporally Associated With an Enterovirus D68 Outbreak: Results of a Nationwide Survey of Acute Flaccid Paralysis in Japan, August-December 2015". Clin. Infect. Dis. 66 (5): 653–664. doi:10.1093/cid/cix860. PMC 5850449. PMID 29028962.
- ↑ Messacar K, Asturias EJ, Hixon AM, Van Leer-Buter C, Niesters H, Tyler KL, Abzug MJ, Dominguez SR (August 2018). "Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality". Lancet Infect Dis. 18 (8): e239–e247. doi:10.1016/S1473-3099(18)30094-X. PMID 29482893. Vancouver style error: initials (help)
- ↑ Chen IJ, Hu SC, Hung KL, Lo CW (September 2018). "Acute flaccid myelitis associated with enterovirus D68 infection: A case report". Medicine (Baltimore). 97 (36): e11831. doi:10.1097/MD.0000000000011831. PMC 6133480. PMID 30200066.
- ↑ "Botulism | Botulism | CDC".
- ↑ McCroskey LM, Hatheway CL (May 1988). "Laboratory findings in four cases of adult botulism suggest colonization of the intestinal tract". J. Clin. Microbiol. 26 (5): 1052–4. PMC 266519. PMID 3290234.
- ↑ Lindström M, Korkeala H (April 2006). "Laboratory diagnostics of botulism". Clin. Microbiol. Rev. 19 (2): 298–314. doi:10.1128/CMR.19.2.298-314.2006. PMC 1471988. PMID 16614251.
- ↑ Brook I (2006). "Botulism: the challenge of diagnosis and treatment". Rev Neurol Dis. 3 (4): 182–9. PMID 17224901.
- ↑ Dimachkie MM, Barohn RJ (May 2013). "Guillain-Barré syndrome and variants". Neurol Clin. 31 (2): 491–510. doi:10.1016/j.ncl.2013.01.005. PMC 3939842. PMID 23642721.
- ↑ Walling AD, Dickson G (February 2013). "Guillain-Barré syndrome". Am Fam Physician. 87 (3): 191–7. PMID 23418763.
- ↑ Gilhus NE (2011). "Lambert-eaton myasthenic syndrome; pathogenesis, diagnosis, and therapy". Autoimmune Dis. 2011: 973808. doi:10.4061/2011/973808. PMC 3182560. PMID 21969911.
- ↑ Krishnan C, Kaplin AI, Deshpande DM, Pardo CA, Kerr DA (May 2004). "Transverse Myelitis: pathogenesis, diagnosis and treatment". Front. Biosci. 9: 1483–99. PMID 14977560.
- ↑ Amato AA, Greenberg SA (December 2013). "Inflammatory myopathies". Continuum (Minneap Minn). 19 (6 Muscle Disease): 1615–33. doi:10.1212/01.CON.0000440662.26427.bd. PMID 24305450.
- ↑ 18.0 18.1 Berger JR, Dean D (2014). "Neurosyphilis". Handb Clin Neurol. 121: 1461–72. doi:10.1016/B978-0-7020-4088-7.00098-5. PMID 24365430.
- ↑ Talukder RK, Sutradhar SR, Rahman KM, Uddin MJ, Akhter H (2011). "Guillian-Barre syndrome". Mymensingh Med J. 20 (4): 748–56. PMID 22081202.
- ↑ Merino-Ramírez MÁ, Bolton CF (2016). "Review of the Diagnostic Challenges of Lambert-Eaton Syndrome Revealed Through Three Case Reports". Can J Neurol Sci. 43 (5): 635–47. doi:10.1017/cjn.2016.268. PMID 27412406.
- ↑ Gilhus NE (2016). "Myasthenia Gravis". N Engl J Med. 375 (26): 2570–2581. doi:10.1056/NEJMra1602678. PMID 28029925.
- ↑ Ozono K (2016). "[Diagnostic criteria for vitamin D-deficient rickets and hypocalcemia-]". Clin Calcium. 26 (2): 215–22. doi:CliCa1602215222 Check
|doi=value (help). PMID 26813501. - ↑ Kamanyire R, Karalliedde L (2004). "Organophosphate toxicity and occupational exposure". Occup Med (Lond). 54 (2): 69–75. PMID 15020723.
- ↑ Pecina CA (2012). "Tick paralysis". Semin Neurol. 32 (5): 531–2. doi:10.1055/s-0033-1334474. PMID 23677663.
- ↑ Bane V, Lehane M, Dikshit M, O'Riordan A, Furey A (2014). "Tetrodotoxin: chemistry, toxicity, source, distribution and detection". Toxins (Basel). 6 (2): 693–755. doi:10.3390/toxins6020693. PMC 3942760. PMID 24566728.
- ↑ Kuntzer T, Hirt L, Bogousslavsky J (1996). "[Neuromuscular involvement and cerebrovascular accidents]". Rev Med Suisse Romande. 116 (8): 605–9. PMID 8848683.
- ↑ Laffont I, Julia M, Tiffreau V, Yelnik A, Herisson C, Pelissier J (2010). "Aging and sequelae of poliomyelitis". Ann Phys Rehabil Med. 53 (1): 24–33. doi:10.1016/j.rehab.2009.10.002. PMID 19944665.
- ↑ West TW (2013). "Transverse myelitis--a review of the presentation, diagnosis, and initial management". Discov Med. 16 (88): 167–77. PMID 24099672.
- ↑ Liu LL, Zheng WH, Tong ML, Liu GL, Zhang HL, Fu ZG; et al. (2012). "Ischemic stroke as a primary symptom of neurosyphilis among HIV-negative emergency patients". J Neurol Sci. 317 (1–2): 35–9. doi:10.1016/j.jns.2012.03.003. PMID 22482824.
- ↑ Ho EL, Marra CM (2012). "Treponemal tests for neurosyphilis--less accurate than what we thought?". Sex Transm Dis. 39 (4): 298–9. doi:10.1097/OLQ.0b013e31824ee574. PMC 3746559. PMID 22421697.
- ↑ Falzarano MS, Scotton C, Passarelli C, Ferlini A (2015). "Duchenne Muscular Dystrophy: From Diagnosis to Therapy". Molecules. 20 (10): 18168–84. doi:10.3390/molecules201018168. PMID 26457695.
- ↑ Filippi M, Preziosa P, Rocca MA (2016). "Multiple sclerosis". Handb Clin Neurol. 135: 399–423. doi:10.1016/B978-0-444-53485-9.00020-9. PMID 27432676.
- ↑ Giang DW, Grow VM, Mooney C, Mushlin AI, Goodman AD, Mattson DH; et al. (1994). "Clinical diagnosis of multiple sclerosis. The impact of magnetic resonance imaging and ancillary testing. Rochester-Toronto Magnetic Resonance Study Group". Arch Neurol. 51 (1): 61–6. PMID 8274111.
- ↑ Riva N, Agosta F, Lunetta C, Filippi M, Quattrini A (2016). "Recent advances in amyotrophic lateral sclerosis". J Neurol. 263 (6): 1241–54. doi:10.1007/s00415-016-8091-6. PMC 4893385. PMID 27025851.
- ↑ Michelle EH, Mammen AL (2015). "Myositis Mimics". Curr Rheumatol Rep. 17 (10): 63. doi:10.1007/s11926-015-0541-0. PMID 26290112.
Template:PNS diseases of the nervous system
de:Lambert-Eaton-Rooke-Syndrom Template:WH Template:WikiDoc Sources