Hydromorphone (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: RISK OF RESPIRATORY DEPRESSION, ABUSE, AND MEDICATION ERRORS
See full prescribing information for complete Boxed Warning.
HYDROMORPHONE HCl INJECTION (high potency formulation) IS FOR USE IN OPIOID-TOLERANT PATIENTS ONLY
|
Overview
Hydromorphone (injection) is an opioid analgesic that is FDA approved for the treatment of pain. There is a Black Box Warning for this drug as shown here. Common adverse reactions include lightheadedness, dizziness, sedation, nausea, vomiting, sweating, flushing, dysphoria, euphoria, dry mouth, and pruritus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Hydromorphone Hydrochloride Injection, USP is indicated for the management of pain in patients where an opioid analgesic is appropriate.
- Hydromorphone Hydrochloride Injection, USP [high potency formulation (HPF)] is indicated for the management of moderate-to-severe pain in opioid-tolerant patients who require higher doses of opioids.
Dosage
General Dosing Considerations
- Take care when prescribing and administering Hydromorphone Hydrochloride Injection (HPF) to avoid dosing errors due to confusion between the different concentrations and between mg and mL, which could result in accidental overdose and death. Take care to ensure the proper dose is communicated and dispensed. When writing prescriptions, include both the total dose in mg and the total volume of the dose.
- Selection of patients and administration of hydromorphone hydrochloride injection (HPF) should be governed by the same principles that apply to the use of similar opioid analgesics to treat patients with acute or chronic pain, and depends upon a comprehensive assessment of the patient. Individualize treatment in every case, using non-opioid analgesics, opioids on an as needed basis and/or combination products, and chronic opioid therapy in a progressive plan of pain management such as outlined by the World Health Organization, the Agency for Healthcare Research and Quality, and the American Pain Society.
- The nature of the pain (severity, frequency, etiology, and pathophysiology), as well as the medical status of the patient, will affect selection of the starting dosage. Opioid analgesics, including hydromorphone hydrochloride injection (HPF), have a narrow therapeutic index in certain patient populations, especially when combined with CNS depressant drugs, and should be reserved for cases where the benefits of opioid analgesia outweigh the known risks.
Individualization of Dosing
- Initiate the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment. Give attention to the following:
- The age, general condition, and medical status of the patient;
- The patient's degree of opioid tolerance;
- The daily dose, potency, and specific characteristics of the opioid the patient has been taking previously;
- Concurrent medications
- The type and severity of the patient's pain
- Risk factors for abuse or addiction; including whether the patient has a previous or Current substance abuse problem, a family history of substance abuse, or a history of Mental illness or depression;
- The balance between pain control and adverse reactions.
- Periodic reassessment after the initial dosing of hydromorphone hydrochloride injection (HPF) is required. If pain management is not satisfactory, and opioid-induced adverse events are tolerable, the hydromorphone dose may be increased gradually. If excessive opioid side effects are observed early in the dosing interval, reduce the hydromorphone hydrochloride dose. If this results in breakthrough pain at the end of the dosing interval, the dosing interval may need to be shortened. Dose titration should be guided more by the need for analgesia and the severity of adverse events than the absolute dose of opioid employed.
Initiation of Therapy in Opioid-Naïve Patients
- Always initiate dosing in opioid-naïve patients using hydromorphone hydrochloride injection. Never administer hydromorphone hydrochloride injection (HPF) to opioid-naïve patients.
Subcutaneous or Intramuscular Administration
- The usual starting dose of hydromorphone hydrochloride injection is 1 mg to 2 mg every 2 to 3 hours as necessary. Depending on the clinical situation, the initial starting dose may be lowered in patients who are opioid naïve. Adjust the dose according to the severity of pain, the severity of adverse events, as well as the patient's underlying disease and age.
Intravenous Administration
- The initial starting dose is 0.2 to 1 mg every 2 to 3 hours. Intravenous administration should be given slowly, over at least 2 to 3 minutes, depending on the dose. Titrate the dose to achieve acceptable analgesia and tolerable adverse events. The initial dose should be reduced in the elderly or debilitated and may be lowered to 0.2 mg.
Hepatic Impairment
- Start patients with hepatic impairment on one-fourth to one-half the usual hydromorphone hydrochloride injection starting dose depending on the extent of impairment.
Renal Impairment
- Start patients with renal impairment on one-fourth to one-half the usual hydromorphone hydrochloride injection starting dose depending on the degree of impairment.
Conversion From Prior Opioid
- Use the equianalgesic dose table below (table1) as a guide to determine the appropriate dose of hydromorphone hydrochloride injection (HPF). Convert the current total daily amount(s) of opioid(s) received to an equivalent total daily dose of hydromorphone hydrochloride injection (HPF) and reduce by one-half due to the possibility of incomplete cross tolerance. Divide the new total amount by the number of doses permitted based on dosing interval (e.g., 8 doses for every-three-hour dosing). Titrate the dose according to the patient's response. For opioids not in table 1, first estimate the daily amount of morphine that is equivalent to the current total daily amount of other opioid(s) received, then use Table1 to find the approximate equivalent total daily dose of hydromorphone hydrochloride injection (HPF).

Hydromorphone Hydrochloride Injection (HPF) (for use in opioid-tolerant patients only) Do not use Hydromorphone Hydrochloride Injection (HPF) for patients who are not tolerant to the respiratory depressant or sedating effects of opioids'.
- Patients considered opioid tolerant are those who are taking at least 60 mg oral morphine/day, 25 mcg transdermal fentanyl/hour, 30 mg oral oxycodone/day, 8 mg oral hydromorphone/day, 25 mg oral oxymorphone/day, or an equianalgesic dose of another opioid for one week or longer.
- Use Hydromorphone Hydrochloride Injection (HPF) ONLY for patients who require the higher concentration and lower total volume of Hydromorphone Hydrochloride Injection (HPF).
- Because of its high concentration, the delivery of precise doses of hydromorphone hydrochloride injection (HPF) may be difficult if low doses of hydromorphone are required. Therefore, use hydromorphone hydrochloride injection (HPF) only if the amount of hydromorphone required can be delivered accurately with this formulation.
- Base the starting dose for hydromorphone hydrochloride injection (HPF) on the prior dose of hydromorphone hydrochloride injection or on the prior dose of an alternate opioid as described above in Section 2.4 Conversion From Prior Opioid.
Administration and Reconstitution
- Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit. A slight yellowish discoloration may develop in hydromorphone hydrochloride injection (HPF) ampules. No loss of potency has been demonstrated. Hydromorphone hydrochloride injection (HPF) are physically compatible and chemically stable for at least 24 hours at 25°C, protected from light in most common large-volume parenteral solutions.
- 500 mg/50 mL Vial
- To use this single dose presentation, do not penetrate the stopper with a syringe. Instead, remove both the aluminum flipseal and rubber stopper in a suitable work area, such as under a laminar flow hood (or equivalent clean air compounding area). The contents may then be withdrawn for preparation of a single, large-volume parenteral solution. Discard any unused portion in an appropriate manner.
DOSAGE FORMS AND STRENGTHS
- Hydromorphone Hydrochloride Injection (high potency formulation) (for use in opioid-tolerant patients only): Each amber ampule and amber single-dose vial contains 10 mg/mL of hydromorphone hydrochloride in a sterile, aqueous solution and is available in 1 mL or 5 mL ampules or in 50 mL single-dose vials†.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydromorphone (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydromorphone (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Hydromorphone (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydromorphone (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydromorphone (injection) in pediatric patients.
Contraindications
- Hydromorphone Hydrochloride Injection [high potency formulation (HPF)] is contraindicated:
- In patients with known hypersensitivity to hydromorphone, hydromorphone salts, any other components of the product, or sulfite-containing medications.
- In any situation where opioids are contraindicated, e.g., in patients with respiratory depression in the absence of resuscitative equipment or in unmonitored settings; or patients with acute or severe bronchial asthma.
- In patients with, or at risk of developing, gastrointestinal obstruction, especially paralytic ileus because hydromorphone diminishes the propulsive peristaltic wave in the gastrointestinal tract and may prolong the obstruction.
- Hydromorphone Hydrochloride Injection [high potency formulation (HPF)] is contraindicated in patients who are not opioid tolerant.
Warnings
|
WARNING: RISK OF RESPIRATORY DEPRESSION, ABUSE, AND MEDICATION ERRORS
See full prescribing information for complete Boxed Warning.
HYDROMORPHONE HCl INJECTION (high potency formulation) IS FOR USE IN OPIOID-TOLERANT PATIENTS ONLY
|
Risk of Medication Errors
- Hydromorphone HCl Injection (HPF) is a 10 mg/mL concentrated solution of hydromorphone, and is intended for use in opioid-tolerant patients only. Patients considered opioid tolerant are those who are taking at least 60 mg oral morphine/day, 25 mcg transdermal fentanyl/hour, 30 mg oral oxycodone/day, 8 mg oral hydromorphone/day, 25 mg oral oxymorphone/day, or an equianalgesic dose of another opioid for one week or longer.
- Do not confuse Hydromorphone HCl Injection (HPF) with standard parenteral formulations of Hydromorphone HCl Injection (1 mg/mL, 2 mg/mL, 4 mg/mL) or other opioids, as overdose and death could result.
- Morphine does not convert to hydromorphone on a mg per mg basis. Use TABLE 1 when converting a patient from morphine to hydromorphone to avoid errors that can lead to overdose or death.
Respiratory Depression
- Respiratory depression is the chief hazard of Hydromorphone HCl injection (HPF). Respiratory depression occurs most frequently in the elderly, in the debilitated, and in those suffering from conditions accompanied by hypoxia or hypercapnia, or upper airway obstruction, in whom even moderate therapeutic doses may dangerously decrease pulmonary ventilation. Respiratory depression is also a particular problem following large initial doses in non opioid-tolerant patients or when opioids are given in conjunction with other agents that depress respiration.
- Use Hydromorphone HCl Injection (HPF) with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale, patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or preexisting respiratory depression. In such patients, even usual therapeutic doses of opioid analgesics may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea. Consider using non-opioid analgesics, and administer Hydromorphone HCl Injection only under careful medical supervision at the lowest effective dose in such patients.
Misuse, Abuse and Diversion of Opioids
- Hydromorphone HCl Injection (HPF) contain hydromorphone, an opioid agonist with an abuse liability similar to morphine, and a Schedule II, controlled substance. Hydromorphone has the potential for being abused, is sought by drug abusers and people with addiction disorders, and is subject to criminal diversion. Diversion of Schedule II products is an act subject to criminal penalty.
- Abuse of Hydromorphone HCl Injection (HPF), poses a hazard of overdose and death. This risk is increased with concurrent abuse of alcohol or other substances. Schedule II opioid agonists have the highest potential for abuse and risk of fatal respiratory depression.
- Hydromorphone HCl Injection (HPF) can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing Hydromorphone HCl Injection (HPF) in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse or diversion.
- Concerns about abuse, addiction, and diversion should not prevent the proper management of pain. Healthcare professionals should contact their State Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Interactions with Alcohol and other CNS Depressants
- The concurrent use of Hydromorphone HCl Injection (HPF) with other central nervous system (CNS) depressants, including but not limited to, other opioids, illicit drugs, sedatives, hypnotics, general anesthetics, phenothiazines, muscle relaxants, other tranquilizers, and alcohol, increases the risk of respiratory depression, hypotension, and profound sedation, potentially resulting in coma or death. Use with caution and in reduced dosages in patients taking CNS depressants.
Neonatal Withdrawal Syndrome
- Infants born to mothers physically dependent on Hydromorphone HCl Injection (HPF) will also be physically dependent and may exhibit signs of withdrawal. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. Neonatal opioid withdrawal syndrome may be life threatening and should be treated according to protocols developed by neonatology experts .
Use in Increased Intracranial Pressure or Head Injury
- The respiratory depressant effects of Hydromorphone HCl Injection (HPF) promote carbon dioxide retention which results in elevation of cerebrospinal fluid pressure. This increase in intracranial pressure may be markedly exaggerated in the presence of head injury, intracranial lesions, or other conditions that predispose patients to increased intracranial pressure.
- Hydromorphone HCl Injection (HPF) may produce effects on pupillary response and consciousness which can obscure the clinical course and neurologic signs of further increase in pressure in patients with head injuries.
Hypotensive Effect
- Hydromorphone HCl Injection (HPF) may cause severe hypotension in patients whose ability to maintain blood pressure is compromised by a depleted blood volume, or a concurrent administration of drugs such as phenothiazines, general anesthetics, or other agents which compromise vasomotor tone.
- Hydromorphone HCl Injection (HPF) may produce orthostatic hypotension in ambulatory patients.
- Administer Hydromorphone HCl Injection (HPF) with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
Sulfites
- Hydromorphone HCl Injection (HPF) contain sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Use in Pancreatic/Biliary Tract Disease and Other Gastrointestinal Conditions
- The administration of Hydromorphone HCl Injection (HPF) may obscure the diagnosis or clinical course in patients with acute abdominal conditions.
Use Hydromorphone HCl Injection (HPF) with caution in patients who are at risk of developing ileus.
- Use Hydromorphone HCl Injection (HPF) with caution in patients with biliary tract disease, including acute pancreatitis, as hydromorphone may cause spasm of the sphincter of Oddi and diminish biliary and pancreatic secretions.
Special Risk Patients
- Give Hydromorphone HCl Injection (HPF) with caution and the initial dose should be reduced in the elderly or debilitated and those with severe impairment of hepatic, pulmonary or renal function; myxedema or hypothyroidism; adrenocortical insufficiency (e.g., Addison's Disease); CNS depression or coma; toxic psychoses; prostatic hypertrophy or urethral stricture; acute alcoholism; delirium tremens; or kyphoscoliosis associated with respiratory depression.
- The administration of opioid analgesics including Hydromorphone HCl Injection (HPF) may aggravate preexisting convulsions in patients with convulsive disorders.
- Hydromorphone HCl Injection (HPF), as with other opioids, may aggravate convulsions in patients with convulsive disorders, and may induce or aggravate seizures in some clinical settings.
- Reports of mild to severe seizures and myoclonus have been reported in severely compromised patients, administered high doses of parenteral hydromorphone.
Use in Drug and Alcohol Dependent Patients
- Use Hydromorphone HCl Injection (HPF) with caution in patients with alcoholism and other drug dependencies due to the increased frequency of opioid tolerance, dependence, and the risk of addiction observed in these patient populations. Abuse of Hydromorphone HCl Injection (HPF) in combination with other CNS depressant drugs can result in serious risk to the patient.
- Hydromorphone HCl Injection (HPF) contain hydromorphone, an opioid with no approved use in the management of addiction disorders. Its proper usage in individuals with drug or alcohol dependence, either active or in remission, is for the management of pain requiring opioid analgesia.
Use in Ambulatory Patients
- Hydromorphone HCl Injection (HPF) may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating machinery). Patients should be cautioned accordingly. Hydromorphone HCl Injection (HPF) may produce orthostatic hypotension in ambulatory patients.
Parenteral Administration
- Hydromorphone HCl Injection may be given intravenously, but the injection should be given very slowly. Rapid intravenous injection of opioid analgesics increases the possibility of side effects such as hypotension and respiratory depression.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Serious adverse reactions associated with Hydromorphone HCl Injection (HPF) include respiratory depression and apnea and, to a lesser degree, circulatory depression, respiratory arrest, shock, and cardiac arrest.
- The following serious adverse reactions described elsewhere in the labeling include:
- Respiratory depression and secondary effects on intracranial pressure
- Hypotension
- Gastrointestinal effects and effects in sphincter of Oddi
- Drug abuse, addiction, and dependence
- Effects on the ability to drive and operate machinery
- The most common adverse effects are lightheadedness, dizziness, sedation, nausea, vomiting, sweating, flushing, dysphoria, euphoria, dry mouth, and pruritus. These effects seem to be more prominent in ambulatory patients and in those not experiencing severe pain.
- Less Frequently Observed Adverse Reactions
- Cardiac disorders: tachycardia, bradycardia, palpitations
- Eye disorders: vision blurred, diplopia, miosis, visual impairment
- Gastrointestinal disorders: constipation, ileus, diarrhea, abdominal pain
- Hepatobiliary disorders: biliary colic
- Metabolism and nutrition disorders: decreased appetite
- Musculoskeletal and connective tissue disorders: muscle rigidity
- Nervous system disorders: headache, tremor, paraesthesia, nystagmus, increased intracranial pressure, syncope, taste alteration, involuntary muscle contractions, presyncope
- Psychiatric disorders: agitation, mood altered, nervousness, anxiety, depression, hallucination, disorientation, insomnia, abnormal dreams
- Renal and urinary disorders: urinary retention, urinary hesitation, antidiuretic effects
- Respiratory, thoracic, and mediastinal disorders: bronchospasm, laryngospasm
- Skin and subcutaneous tissue disorders: injection site pain, urticaria, rash, hyperhidrosis
- Vascular disorders: flushing, hypotension, hypertension
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of hydromorphone. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: anaphylactic reactions, confusional state, convulsions, drowsiness, dyskinesia, dyspnea, erectile dysfunction, fatigue, hepatic enzymes increased, hyperalgesia, hypersensitivity reaction, injection site reactions, lethargy, myoclonus, oropharyngeal swelling, peripheral edema, and somnolence.
Drug Interactions
Drug Interactions with other CNS Depressants
- Hydromorphone HCl Injection (HPF) should be used with caution and in reduced dosages when administered to patients concurrently receiving other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, centrally acting antiemetics, tranquilizers, and alcohol because respiratory depression, hypotension, and profound sedation or coma may result.
- When such combined therapy is contemplated, the dose of one or both agents should be reduced. Opioid analgesics, including Hydromorphone HCl Injection (HPF), may enhance the action of neuromuscular blocking agents and produce an increased degree of respiratory depression.
Interactions with Mixed Agonist/Antagonist Opioid Analgesics
- Agonist/antagonist analgesics (e.g., pentazocine, nalbuphine, and butorphanol) and partial agonist analgesics (buprenorphine) should be administered with caution to a patient who has received or is receiving a course of therapy with a pure opioid agonist analgesic such as Hydromorphone HCl Injection (HPF). In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of Hydromorphone HCl Injection (HPF) and/or may precipitate withdrawal symptoms in these patients.
Monoamine Oxidase Inhibitors (MAOIs)
- MAOIs may potentiate the action of Hydromorphone HCl Injection (HPF). Allow at least 14 days after stopping treatment with MAOIs before initiating treatment with Hydromorphone HCl Injection (HPF).
Anticholinergics
- Anticholinergics or other medications with anticholinergic activity when used concurrently with Hydromorphone HCl Injection (HPF) may result in increased risk of urinary retention and severe constipation, which may lead to paralytic ileus.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Teratogenic Effects
- Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. Hydromorphone crosses the placenta. Hydromorphone HCl Injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- No effects on teratogenicity or embryotoxicity were observed in pregnant rats given oral doses up to 7 mg/kg/day which is 3-fold higher than the human dose of 24 mg Hydromorphone HCl Injection (HPF) (4 mg every 4 hours), on a body surface area basis. Hydromorphone administration to pregnant Syrian hamsters and CF-1 mice during major organ development revealed teratogenic effects likely the result of maternal toxicity associated with sedation and hypoxia. In Syrian hamsters given single subcutaneous doses from 14 to 258 mg/kg during organogenesis (gestation days 8 to 10), doses ≥ 19 mg/kg of hydromorphone produced skull malformations (exencephaly and cranioschisis). In CF-1 mice, continuous infusion of hydromorphone (≥ 15 mg/kg over 24 hours) via implanted osmotic pumps during organogenesis (gestation days 7 to 10) produced soft tissue malformations (cryptorchidism, cleft palate, malformed ventricles and retina), and skeletal variations (split supraoccipital, checkerboard and split sternebrae, delayed ossification of the paws and ectopic ossification sites). The malformations and variations observed in the hamsters and mice were observed at doses approximately 6-fold and 3-fold higher, respectively, than the human dose of 24 mg Hydromorphone HCl Injection (4 mg every 4 hours), on a body surface area basis.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hydromorphone (injection) in women who are pregnant.
Labor and Delivery
- Hydromorphone HCl should be used with caution during labor. Opioids cross the placenta and may produce respiratory depression and physiologic effects in neonates. Sinusoidal fetal heart rate patterns may occur with the use of opioid analgesics.
- Occasionally, opioid analgesics, including Hydromorphone HCl Injection (HPF), may prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilatation, which tends to shorten labor.
- Opioid analgesics, including Hydromorphone HCl Injection (HPF), may cause respiratory depression in the newborn. Closely observe neonates whose mothers received opioid analgesics during labor for signs of respiratory depression. Have a specific opioid antagonist, such as naloxone or nalmefene, available for reversal of opioid-induced respiratory depression in the neonate.
- Neonates whose mothers have been taking opioids chronically may also exhibit withdrawal signs, either at birth or in the nursery, because they have developed physical dependence. This is not, however, synonymous with addiction. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening and should be treated according to protocols developed by neonatology experts.
- The effect of Hydromorphone HCl, if any, on the later growth, development, and functional maturation of the child is unknown.
Nursing Mothers
- Low levels of opioid analgesics have been detected in human milk. As a general rule, nursing should not be undertaken while a patient is receiving Hydromorphone HCl Injection (HPF) since it, and other drugs in this class, may be excreted in the milk.
Pediatric Use
- The safety and effectiveness of Hydromorphone HCl Injection (HPF) in pediatric patients has not been established.
Geriatic Use
- Clinical studies of Hydromorphone HCl Injection (HPF) did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Respiratory depression is the chief risk in elderly or debilitated patients, usually the result of large initial doses in non-opioid-tolerant patients. Titration in these patients should proceed cautiously
Gender
There is no FDA guidance on the use of Hydromorphone (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hydromorphone (injection) with respect to specific racial populations.
Renal Impairment
- The pharmacokinetics of hydromorphone following an oral administration of hydromorphone at a single 4 mg dose (2 mg hydromorphone immediate-release tablets) are affected by renal impairment. Mean exposure to hydromorphone (Cmax and AUC0-∞) is increased by 2-fold in patients with moderate (CLcr = 40 to 60 mL/min) renal impairment and increased by 4-fold in patients with severe (CLcr < 30 mL/min) renal impairment compared with normal subjects (CLcr > 80 mL/min). In addition, in patients with severe renal impairment, hydromorphone appeared to be more slowly eliminated with a longer terminal elimination half-life (40 hr) compared to patients with normal renal function (15 hr). Start patients with renal impairment on one-fourth to one-half the usual starting dose depending on the degree of impairment. Patients with renal impairment should be closely monitored during dose titration
Hepatic Impairment
- The pharmacokinetics of hydromorphone following an oral administration of hydromorphone at a single 4 mg dose (2 mg hydromorphone immediate-release tablets) are affected by hepatic impairment. Mean exposure to hydromorphone (Cmax and AUC∞) is increased 4-fold in patients with moderate (Child-Pugh Group B) hepatic impairment compared with subjects with normal hepatic function. Due to increased exposure of hydromorphone, patients with moderate hepatic impairment should be started at one-fourth to one-half the recommended starting dose depending on the degree of hepatic dysfunction and closely monitored during dose titration. The pharmacokinetics of hydromorphone in patients with severe hepatic impairment has not been studied. A further increase in Cmax and AUC of hydromorphone in this group is expected and should be taken into consideration when selecting a starting do
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hydromorphone (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hydromorphone (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Hydromorphone (injection) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Hydromorphone (injection) in the drug label.
Overdosage
Signs and Symptoms
- Signs and symptoms of acute overdosage with Hydromorphone HCl Injection (HPF) include: respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, bradycardia, hypotension, partial or complete airway obstruction, atypical snoring, apnea, circulatory collapse, cardiac arrest, and death.
- Hydromorphone may cause miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis, rather than miosis, may be seen with hypoxia in overdose situations.
Treatment
- In the treatment of overdosage, primary attention should be given to the reestablishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen, vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
- The opioid antagonist, naloxone, is a specific antidote against respiratory depression which may result from overdosage, or unusual sensitivity to Hydromorphone HCl Injection (HPF). Therefore, an appropriate dose of this antagonist should be administered, preferably by the intravenous route, simultaneously with efforts at respiratory resuscitation. Naloxone should not be administered in the absence of clinically significant respiratory or circulatory depression. Naloxone should be administered cautiously to persons who are known, or suspected, to be physically dependent on Hydromorphone HCl Injection (HPF). In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute withdrawal syndrome.
- Since the duration of action of Hydromorphone HCl Injection (HPF) may exceed that of the antagonist, the patient should be kept under continued surveillance; repeated doses of the antagonist may be required to maintain adequate respiration. Apply other supportive measures when indicated.
Pharmacology
Mechanism of Action
- The precise mode of analgesic action of opioid analgesics is unknown. However, specific CNS opiate receptors have been identified. Opioids are believed to express their pharmacological effects by combining with these receptors.
- Hydromorphone hydrochloride is a mu-opioid receptor agonist whose principal therapeutic action is analgesia. Other members of the class known as opioid agonists include substances such as morphine, oxycodone, fentanyl, codeine, hydrocodone, and oxymorphone.
Central Nervous System
- Pharmacological effects of opioid agonists include anxiolysis, euphoria, feelings of relaxation, and cough suppression, as well as analgesia.
- Hydromorphone produces respiratory depression by direct effect on brain stem respiratory centers. The mechanism of respiratory depression also involves a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension.
- Hydromorphone causes miosis. Pinpoint pupils are a common sign of opioid overdose but are not pathognomonic (pontine lesions of hemorrhagic or ischemic origin may produce similar findings).
Gastrointestinal Tract and Other Smooth Muscle
- Gastric, biliary and pancreatic secretions are decreased by opioids such as hydromorphone. Hydromorphone causes a reduction in motility associated with an increase in tone in the gastric antrum and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, and tone may be increased to the point of spasm. The end result is constipation. Hydromorphone can cause a marked increase in biliary tract pressure as a result of spasm of the sphincter of Oddi.
Cardiovascular System
- Hydromorphone may produce hypotension as a result of either peripheral vasodilation, release of histamine, or both. Other manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, and red eyes.
- Effects on the myocardium after intravenous administration of opioids are not significant in normal persons, vary with different opioid analgesic agents and vary with the hemodynamic state of the patient, state of hydration and sympathetic drive.
Endocrine System
- Opioids may influence the hypothalamic-pituitary-adrenal or -gonadal axes. Some changes that can be seen include an increase in serum prolactin, and decreases in plasma cortisol and testosterone. Clinical signs and symptoms may be manifest from these hormonal changes.
Immune System
- In vitro and animal studies indicate that opioids have a variety of effects on immune functions. The clinical significance of these findings is unknown.
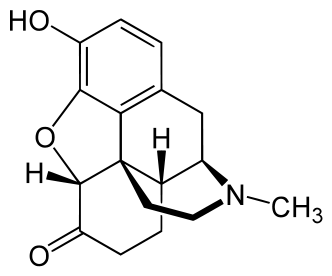
Structure
- Hydromorphone hydrochloride injection, USP, a hydrogenated ketone of morphine, is an opioid analgesic. The chemical name of hydromorphone hydrochloride is 4,5∝-epoxy-3 hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula is:

Hydromorphone hydrochloride injection, USP [high potency formulation (HPF)] is available as a sterile, aqueous solution in AMBER ampules and in AMBER, single-dose vials for intravenous, subcutaneous, or intramuscular administration. Each ampule and single-dose vial contains 10 mg/mL of hydromorphone hydrochloride with 0.2% sodium citrate and 0.2% citric acid added as a buffer to maintain a pH of between 3.5 and 5.5. The single dose vials are capped with stoppers not containing natural rubber latex.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Hydromorphone (injection) in the drug label.
Pharmacokinetics
Distribution
- At therapeutic plasma levels, hydromorphone is approximately 8 to 19% bound to plasma proteins. After an intravenous bolus dose, the steady state of volume of distribution [mean (%CV)] is 302.9 (32%) liters.
Metabolism
- Hydromorphone is extensively metabolized via glucuronidation in the liver, with greater than 95% of the dose metabolized to hydromorphone-3-glucuronide along with minor amounts of 6-hydroxy reduction metabolites.
Elimination
- Only a small amount of the hydromorphone dose is excreted unchanged in the urine. Most of the dose is excreted as hydromorphone-3-glucuronide along with minor amounts of 6-hydroxy reduction metabolites. The systemic clearance is approximately 1.96 (20%) liters/minute. The terminal elimination half-life of hydromorphone after an intravenous dose is about 2.3 hours.
Special Populations
Hepatic Impairment
- After oral administration of hydromorphone at a single 4 mg dose (2 mg hydromorphone immediate-release tablets), mean exposure to hydromorphone (Cmax and AUC∞) is increased 4-fold in patients with moderate (Child-Pugh Group B) hepatic impairment compared with subjects with normal hepatic function. Patients with moderate hepatic impairment should be started at onefourth to one-half the recommended starting dose and closely monitored during dose titration. The pharmacokinetics of hydromorphone in patients with severe hepatic impairment has not been studied. A further increase in Cmax and AUC of hydromorphone in this group is expected and should be taken into consideration when selecting a starting dose.
Renal Impairment
- The pharmacokinetics of hydromorphone following an oral administration of hydromorphone at a single 4 mg dose (2 mg hydromorphone immediate-release tablets) are affected by renal impairment. Mean exposure to hydromorphone (Cmax and AUC0-∞) is increased by 2-fold in patients with moderate (CLcr = 40 to 60 mL/min) renal impairment and increased by 4-fold in patients with severe (CLcr < 30 mL/min) renal impairment compared with normal subjects (CLcr > 80 mL/min). In addition, in patients with severe renal impairment, hydromorphone appeared to be more slowly eliminated with a longer terminal elimination half-life (40 hr) compared to patients with normal renal function (15 hr). Start patients with renal impairment on one-fourth to one-half the usual starting dose depending on the degree of impairment. Patients with renal impairment should be closely monitored during dose titration.
Pediatrics
- Pharmacokinetics of hydromorphone have not been evaluated in children.
Geriatric
- In the geriatric population, age has no effect on the pharmacokinetics of hydromorphone.
Gender
- Gender has little effect on the pharmacokinetics of hydromorphone. Females appear to have a higher Cmax (25%) than males with comparable AUC0-24 values. The difference observed in Cmax may not be clinically relevant.
Race
- The effect of race on hydromorphone pharmacokinetics has not been studied.
Pregnancy and Nursing Mothers
- Hydromorphone crosses the placenta. Hydromorphone is also found in low levels in breast milk, and may cause respiratory compromise in newborns when administered during labor or delivery.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Long term studies in animals to evaluate the carcinogenic potential of hydromorphone have not been conducted.
Mutagenesis
- Hydromorphone was not mutagenic in the in vitro bacterial reverse mutation assay (Ames assay). Hydromorphone was not clastogenic in either the in vitro human lymphocyte chromosome aberration assay or the in vivo mouse micronucleus assay.
Impairment of Fertility
- No effects on fertility, reproductive performance, or reproductive organ morphology were observed in male or female rats given oral doses up to 7 mg/kg/day which is 3-fold higher than the human dose of 24 mg hydromorphone hydrochloride injection (4 mg every 4 hours), on a body surface area basis.
Clinical Studies
- Analgesic effects of single doses of Hydromorphone Hydrochloride Oral Liquid administered to patients with post-surgical pain have been studied in double-blind controlled trials. In one study, both 5 mg and 10 mg of hydromorphone Hydrochloride Oral Liquid provided significantly more analgesia than placebo.
How Supplied
- Hydromorphone Hydrochloride Injection, USP (High Potency Formulation) is available in AMBER ampules, and AMBER single dose vials. Each 1 mL of sterile solution contains 10 mg of hydromorphone hydrochloride with 0.2% sodium citrate and 0.2% citric acid. No added preservative.
- Hydromorphone hydrochloride injection (High Potency Formulation) is supplied as follows:
- NDC 17478-540-01 Box of ten 1 mL (10 mg) ampules
- NDC 17478-540-05 Box of ten 5 mL (50 mg) ampules
- NDC 17478-540-50 One 50 mL (500 mg) Single-dose Vial†
Storage
Store at 20° to 25°C (68° to 77°F).
Images
Drug Images
{{#ask: Page Name::Hydromorphone (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel





{{#ask: Label Page::Hydromorphone (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Hydromorphone (injection) in the drug label.
Precautions with Alcohol
- Alcohol-Hydromorphone (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- HYDROMORPHONE HYDROCHLORIDE®[3]
Look-Alike Drug Names
- A® — B®[4]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Coda BA, Rudy AC, Archer SM, Wermeling DP (July 2003). "Pharmacokinetics and bioavailability of single-dose intranasal hydromorphone hydrochloride in healthy volunteers". Anesth. Analg. 97 (1): 117–23, table of contents. doi:10.1213/01.ANE.0000066311.40978.4F. PMID 12818953.
- ↑ Vallner JJ, Stewart JT, Kotzan JA, Kirsten EB, Honigberg IL (April 1981). "Pharmacokinetics and bioavailability of hydromorphone following intravenous and oral administration to human subjects". J Clin Pharmacol. 21 (4): 152–6. doi:10.1002/j.1552-4604.1981.tb05693.x. PMID 6165742.
- ↑ "hydromorphone hydrochloride injection".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Hydromorphone (injection)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Hydromorphone (injection) |Label Name=Hydromorphone (injection)11.png
}}
{{#subobject:
|Label Page=Hydromorphone (injection) |Label Name=Hydromorphone (injection)11.png
}}