Gadobutrol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
NEPHROGENIC SYSTEMIC FIBROSIS (NSF):
|
Overview
Gadobutrol is a gadolinium-based contrast agent that is FDA approved for the procedure of magnetic resonance imaging (MRI) to detect and visualize areas with disrupted blood brain barrier (BBB) and/or abnormal vascularity of the central nervous system in adult and pediatric patients (including term neonates and to assess the presence and extent of malignant breast disease. There is a Black Box Warning for this drug as shown here. Common adverse reactions include headache, nausea, and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Magnetic Resonance Imaging (MRI) of the Central Nervous System (CNS)
Gadavist is indicated for use with magnetic resonance imaging (MRI) in adult to detect and visualize areas with disrupted blood brain barrier (BBB) and/or abnormal vascularity of the central nervous system.
MRI of the Breast
Gadavist is indicated for use with MRI to assess the presence and extent of malignant breast disease.
Recommended Dose
- The recommended dose of Gadavist for adult is 0.1 mL/kg body weight (0.1 mmol/kg). Refer to Table 1 to determine the volume to be administered.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gadobutrol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gadobutrol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Magnetic Resonance Imaging (MRI) of the Central Nervous System (CNS)
Gadavist is indicated for use with magnetic resonance imaging (MRI) in pediatric patients (including term neonates) to detect and visualize areas with disrupted blood brain barrier (BBB) and/or abnormal vascularity of the central nervous system.
Recommended Dose
- The recommended dose of Gadavist for pediatric patients (including term neonates) is 0.1 mL/kg body weight (0.1 mmol/kg). Refer to Table 1 to determine the volume to be administered.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gadobutrol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gadobutrol in pediatric patients.
Contraindications
- Gadavist is contraindicated in patients with history of severe hypersensitivity reactions to Gadavist.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
NEPHROGENIC SYSTEMIC FIBROSIS (NSF):
|
Precautions
- Nephrogenic Systemic Fibrosis
- Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR < 30 mL/min/1.73m2) as well as patients with acute kidney injury. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30 to 59 mL/min/1.73m2) and little, if any, for patients with chronic, mild kidney disease (GFR 60 to 89 mL/min/1.73m2). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. Report any diagnosis of NSF following Gadavist administration to Bayer Healthcare (1-888-842-2937) or FDA (1-800-FDA-1088 or www.fda.gov/medwatch).
- Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (for example, age > 60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing.
- Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. For patients at highest risk for NSF, do not exceed the recommended Gadavist dose and allow a sufficient period of time for elimination of the drug prior to re-administration. For patients receiving hemodialysis, consider the prompt initiation of hemodialysis following the administration of a GBCA in order to enhance the contrast agent’s elimination. The usefulness of hemodialysis in the prevention of NSF is unknown.
- Hypersensitivity Reactions
- Anaphylactic and other hypersensitivity reactions with cardiovascular, respiratory or cutaneous manifestations, ranging from mild to severe, including death, have uncommonly occurred following Gadavist administration.
- Before Gadavist administration, assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders. These patients may have an increased risk for a hypersensitivity reaction to Gadavist.
- Administer Gadavist only in situations where trained personnel and therapies are promptly available for the treatment of hypersensitivity reactions, including personnel trained in resuscitation.
- Most hypersensitivity reactions to Gadavist have occurred within half an hour after administration. Delayed reactions can occur up to several days after administration. Observe patients for signs and symptoms of hypersensitivity reactions during and following Gadavist administration.
- Acute Kidney Injury
- In patients with chronic renal impairment, acute kidney injury sometimes requiring dialysis has been observed with the use of some GBCAs. Do not exceed the recommended dose; the risk of acute kidney injury may increase with higher than recommended doses.
- Extravasation and Injection Site Reactions
- Ensure catheter and venous patency before the injection of Gadavist. Extravasation into tissues during Gadavist administration may result in moderate irritation.
- Overestimation of Extent of Malignant Disease in MRI of the Breast
- Gadavist MRI of the breast overestimated the histologically confirmed extent of malignancy in the diseased breast in up to 50% of the patients.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The adverse reactions described in this section reflect Gadavist exposure in 6,330 subjects (including 184 pediatric patients, ages 0 to 17 years) with the majority receiving the recommended dose. Approximately 50% of the subjects were male and the ethnic distribution was 60% Caucasian, 30% Asian, 6% Hispanic, 2% Black, and 3% patients of other ethnic groups. The average age was 55 years (range from1 week to 93 years).
- Overall, approximately 4% of subjects reported one or more adverse reactions during a follow-up period that ranged from 24 hours to 7 days after Gadavist administration.
- Adverse reactions associated with the use of Gadavist were usually mild to moderate in severity and transient in nature.
- Table 2 lists adverse reactions that occurred in ≥ 0.1% subjects who received Gadavist.
- Adverse reactions that occurred with a frequency of < 0.1% in subjects who received Gadavist include: loss of consciousness, convulsion, parosmia, tachycardia, palpitation, dry mouth, malaise and feeling cold.
Postmarketing Experience
- The following additional adverse reactions have been reported during postmarketing use of Gadavist. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiac arrest
- Nephrogenic Systemic Fibrosis (NSF)
- Hypersensitivity reactions (anaphylactic shock, circulatory collapse, respiratory arrest, pulmonary edema, bronchospasm, cyanosis, oropharyngeal swelling, laryngeal edema, blood pressure increased, chest pain, angioedema, conjunctivitis, hyperhidrosis, cough, sneezing, burning sensation, and pallor)
Drug Interactions
There is limited information regarding Gadobutrol Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Risk Summary
- There are no adequate and well-controlled studies of Gadavist in pregnant women. GBCAs cross the human placenta. Limited human data on exposure to GBCAs during pregnancy does not show adverse effects in exposed neonates. Animal reproductive studies were conducted. Embryolethality but no teratogenic effects were observed in monkeys, rabbits and rats. Use Gadavist during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Animal Data
- Embryolethality was observed when gadobutrol was administered intravenously to monkeys during organogenesis at doses 8 times the recommended single human dose (based on body surface area); gadobutrol was not maternally toxic or teratogenic at this dose. Embryolethality and retardation of embryonal development also occurred in pregnant rats receiving maternally toxic doses of gadobutrol (≥ 7.5 mmol/kg body weight; equivalent to12 times the human dose based on body surface area) and in pregnant rabbits (≥ 2.5 mmol/kg body weight; equivalent to 8 times the recommended human dose based on body surface area). In rabbits, this finding occurred without evidence of pronounced maternal toxicity and with minimal placental transfer (0.01% of the administered dose detected in the fetuses).
- Gadavist was not teratogenic when given intravenously to monkeys during organogenesis at doses up to 8 times the recommended single human dose (based on body surface area) but was embryolethal at that dose. Because pregnant animals received repeated daily doses of Gadavist, their overall exposure was significantly higher than that achieved with the standard single dose administered to humans.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Gadobutrol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Gadobutrol during labor and delivery.
Nursing Mothers
- It is not known whether Gadavist is present in human milk. However, reports on use of other GBCAs indicate that 0.01 to 0.04% of the maternal gadolinium dose is present in breast milk and there is limited GBCA gastrointestinal absorption in the breast-fed infant. In rat lactation studies, gadobutrol was present in milk in amounts less than 0.1% of the dose intravenously administered and the gastrointestinal absorption is poor (approximately 5% of the dose orally administered was excreted in the urine). In lactating rats receiving 0.5 mmol/kg of intravenous [153Gd]-gadobutrol, 0.01% of the total administered radioactivity was transferred to the pup via maternal milk, within 3 hours after administration.
- A lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk up to18 hours after Gadavist administration in order to minimize exposure to a breastfed infant.
Pediatric Use
- The safety and effectiveness of Gadavist have been established in pediatric patients born at 37 weeks gestation or later based on imaging and pharmacokinetic data in 138 patients ages 2 to 17 years and 44 patients ages 0 to less than 2 years and extrapolation from adult data. The frequency, type, and severity of adverse reactions in pediatric patients were similar to adverse reactions in adults. No dose adjustment according to age is necessary in pediatric patients. The safety and effectiveness of Gadavist have not been established in premature infants.
- NSF Risk
- No case of NSF associated with Gadavist or any other GBCA has been identified in pediatric patients ages 6 years and younger. Pharmacokinetic studies suggest that clearance of Gadavist is similar in pediatric patients and adults, including pediatric patients age younger than 2 years. No increased risk factor for NSF has been identified in juvenile animal studies of gadobutrol. Normal estimated GFR (eGFR) is around 30 mL/min/1.73m2 at birth and increases to mature levels around 1 year of age, reflecting growth in both glomerular function and relative body surface area. Clinical studies in pediatric patients younger than 1 year of age have been conducted in patients with the following minimum eGFR: 31 mL/min/1.73m2 (age 2 to 7 days), 38 mL/min/1.73m2 (age 8 to 28 days), 62 mL/min/1.73m2 (age 1 to 6 months), and 83 mL/min/1.73m2 (age 6 to 12 months).
- Juvenile Animal Data
- Single and repeat-dose toxicity studies in neonatal and juvenile rats did not reveal findings suggestive of a specific risk for use in pediatric patients including term neonates and infants.
Geriatic Use
- In clinical studies of Gadavist, 1,377 patients were 65 years of age and over, while 104 patients were 80 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, use of Gadavist in elderly patients should be cautious, reflecting the greater frequency of impaired renal function and concomitant disease or other drug therapy. No dose adjustment according to age is necessary in this population.
Gender
There is no FDA guidance on the use of Gadobutrol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Gadobutrol with respect to specific racial populations.
Renal Impairment
- Prior to administration of Gadavist, screen all patients for renal dysfunction by obtaining a history and/or laboratory tests. No dosage adjustment is recommended for patients with renal impairment.
- Gadavist can be removed from the body by hemodialysis.
Hepatic Impairment
There is no FDA guidance on the use of Gadobutrol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Gadobutrol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Gadobutrol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
- Gadavist is formulated at a higher concentration (1 mmol/mL) compared to certain other gadolinium based contrast agents, resulting in a lower volume of administration. Closely examine Table 1 to determine the volume to be administered.
- Use sterile technique when preparing and administering Gadavist.
- Administer Gadavist as an intravenous bolus injection, manually or by power injector, at a flow rate of approximately 2 mL/second.
- Follow Gadavist injection with a normal saline flush to ensure complete administration of the contrast.
- Contrast-enhanced MRI can commence immediately following contrast administration.
Monitoring
There is limited information regarding Monitoring of Gadobutrol in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Gadobutrol in the drug label.
Overdosage
Acute Overdose
- The maximum dose of Gadavist tested in healthy volunteers, 1.5 mL/kg body weight (1.5 mmol/kg) (15 times the recommended dose), was tolerated in a manner similar to lower doses. Gadavist can be removed by hemodialysis.
Chronic Overdose
There is limited information regarding Chronic Overdose of Gadobutrol in the drug label.
Pharmacology
| |
Gadobutrol
| |
| Systematic (IUPAC) name | |
| gadolinium(III) 2,2',2''-(10-((2R,3S)-1,3,4-trihydroxybutan-2-yl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate | |
| Identifiers | |
| CAS number | |
| ATC code | V08 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 604.710 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
C(US) |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | IV |
Mechanism of Action
- In MRI, visualization of normal and pathological tissue depends in part on variations in the radiofrequency signal intensity that occurs with:
- Differences in proton density
- Differences of the spin-lattice or longitudinal relaxation times (T 1)
- Differences in the spin-spin or transverse relaxation time (T 2)
- When placed in a magnetic field, Gadavist shortens the T1 and T2 relaxation times. The extent of decrease of T1 and T2 relaxation times, and therefore the amount of signal enhancement obtained from Gadavist, is based upon several factors including the concentration of Gadavist in the tissue, the field strength of the MRI system, and the relative ratio of the longitudinal and transverse relaxation times. At the recommended dose, the T1 shortening effect is observed with greatest sensitivity in T1-weighted magnetic resonance sequences. In T2*-weighted sequences the induction of local magnetic field inhomogeneities by the large magnetic moment of gadolinium and at high concentrations (during bolus injection) leads to a signal decrease.
Structure
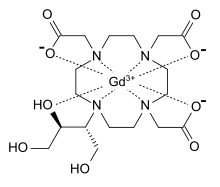
- Gadavist (gadobutrol) injection is a paramagnetic macrocyclic contrast agent administered for magnetic resonance imaging. The chemical name for gadobutrol is 10–[(1SR,2RS)–2,3–dihydroxy–1–hydroxymethylpropyl]–1,4,7,10–tetraazacyclododecane–1,4,7–triacetic acid, gadolinium complex. Gadobutrol has a molecular formula of C18H31GdN4O9 and a molecular weight of 604.72.
- Gadavist is a sterile, clear, colorless to pale yellow solution containing 604.72 mg gadobutrol per mL (equivalent to 1 mmol/mL) as the active ingredient and the excipients calcobutrol sodium, trometamol, hydrochloric acid (for pH adjustment) and water for injection. Gadavist contains no preservatives.
- The main physicochemical properties of Gadavist (1 mmol/mL solution for injection) are listed below:
- The thermodynamic stability constants for gadobutrol (log Ktherm and log Kcond at pH 7.4) are 21.8 and 15.3, respectively.
Pharmacodynamics
- Gadavist leads to distinct shortening of the relaxation times even in low concentrations. At pH 7, 37°C and 1.5 T, the relaxivity (r1) - determined from the influence on the relaxation times (T1) of protons in plasma - is 5.2 L/(mmol·sec) and the relaxivity (r2) - determined from the influence on the relaxation times (T2) - is 6.1 L/(mmol·sec). These relaxivities display only slight dependence on the strength of the magnetic field. The T1 shortening effect of paramagnetic contrast agents is dependent on concentration and r1 relaxivity (see Table 3). This may improve tissue visualization.
- Compared to 0.5 molar gadolinium-based contrast agents, the higher concentration of Gadavist results in half the volume of administration and a more compact contrast bolus.
- Gadavist is a highly water-soluble, extremely hydrophilic compound with a partition coefficient between n-butanol and buffer at pH 7.6 of about 0.006.
Pharmacokinetics
- Distribution
- After intravenous administration, gadobutrol is rapidly distributed in the extracellular space. After a gadobutrol dose of 0.1 mmol/kg body weight, an average level of 0.59 mmol gadobutrol/L was measured in plasma 2 minutes after the injection and 0.3 mmol gadobutrol/L 60 minutes after the injection. Gadobutrol does not display any particular protein binding. In rats, gadobutrol does not penetrate the intact blood-brain barrier.
- Metabolism
- Gadobutrol is not metabolized.
- Elimination
- Values for AUC, body weight normalized plasma clearance and half-life are given in Table 4, below.
- Gadobutrol is excreted in an unchanged form via the kidneys. In healthy subjects, renal clearance of gadobutrol is 1.1 to 1.7 mL/(min∙kg) and thus comparable to the renal clearance of inulin, confirming that gadobutrol is eliminated by glomerular filtration.
- Within two hours after intravenous administration more than 50% and within 12 hours more than 90% of the given dose is eliminated via the urine. Extra-renal elimination is negligible.
- Specific Populations
- Gender
- Gender has no clinically relevant effect on the pharmacokinetics of gadobutrol.
- Geriatric
- A single IV dose of 0.1 mmol/kg Gadavist was administered to 15 elderly and 16 non-elderly subjects. AUC was slightly higher and clearance slightly lower in elderly subjects as compared to non-elderly subjects.
- Pediatric
- The pharmacokinetics of gadobutrol were evaluated in two studies in a total of 130 patients age 2 to less than 18 years and in 43 patients less than 2 years of age (including term neonates). Patients received a single intravenous dose of 0.1 mmol/kg of Gadavist. The pharmacokinetic profile of gadobutrol in pediatric patients is similar to that in adults, resulting in similar values for AUC, body weight normalized plasma clearance, as well as elimination half-life. Approximately 99% (median value) of the dose was recovered in urine within 6 hours (this information was derived from the 2 to less than 18 year old age group).
- Renal Impairment
- In patients with impaired renal function, the serum half-life of gadobutrol is prolonged and correlated with the reduction in creatinine clearance.
- After intravenous injection of 0.1 mmol gadobutrol/kg body weight, the elimination half-life was 5.8 ± 2.4 hours in mild to moderately impaired patients (80 > CLCR > 30 mL/min) and 17.6 ± 6.2 hours in severely impaired patients not on dialysis (CLCR < 30 mL/min). The mean AUC of gadobutrol in patients with normal renal function was 1.1 ± 0.1 mmol∙h/L, compared to 4.0 ± 1.8 mmol∙h/L in patients with mild to moderate renal impairment and 11.5 ± 4.3 mmol∙h/L in patients with severe renal impairment.
- Complete recovery in the urine was seen in patients with mild or moderate renal impairment within 72 hours. In patients with severely impaired renal function about 80% of the administered dose was recovered in the urine within 5 days.
- For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of Gadavist in order to enhance the contrast agent’s elimination. Sixty-eight percent (68%) of gadobutrol is removed from the body after the first dialysis, 94% after the second dialysis, and 98% after the third dialysis session.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No carcinogenicity studies of gadobutrol have been conducted.
- Gadobutrol was not mutagenic in in vitro reverse mutation tests in bacteria, in the HGPRT (hypoxanthine-guanine phosphoribosyl transferase) test using cultured Chinese hamster V79 cells, or in chromosome aberration tests in human peripheral blood lymphocytes, and was negative in an in vivo micronucleus test in mice after intravenous injection of 0.5 mmol/kg.
- Gadobutrol had no effect on fertility and general reproductive performance of male and female rats when given in doses 12.2 times the human equivalent dose (based on body surface area).
Animal Toxicology and/or Pharmacology
- Local intolerance reactions, including moderate irritation associated with infiltration of inflammatory cells was observed after paravenous administration to rabbits, suggesting the possibility of occurrence of local irritation if the contrast medium leaks around veins in a clinical setting.
Clinical Studies
MRI of the CNS
- Patients referred for MRI of the central nervous system with contrast were enrolled in two clinical trials that evaluated the visualization characteristics of lesions. In both studies, patients underwent a baseline, pre-contrast MRI prior to administration of Gadavist at a dose of 0.1 mmol/kg, followed by a post-contrast MRI. In study A, patients also underwent an MRI before and after the administration of gadoteridol. The studies were designed to demonstrate superiority of Gadavist MRI to non-contrast MRI for lesion visualization. For both studies, pre-contrast and pre-plus-post contrast images (paired images) were independently evaluated by three readers for contrast enhancement and border delineation using a scale of 1 to 4, and for internal morphology using a scale of 1 to 3 (Table 5). Lesion counting was also performed to demonstrate non-inferiority of paired Gadavist image sets to pre-contrast MRI. Readers were blinded to clinical information.
- Efficacy was determined in 657 subjects. The average age was 49 years (range 18 to 85 years) and 42% were male. The ethnic representations were 39% Caucasian, 4% Black, 16% Hispanic, 38% Asian, and 3% of other ethnic groups.
- Table 6 shows a comparison of visualization results between paired images and pre-contrast images. Gadavist provided a statistically significant improvement for each of the three lesion visualization parameters when averaged across three independent readers for each study.
- Performances of Gadavist and gadoteridol for visualization parameters were similar. Regarding the number of lesions detected, study B met the prespecified noninferiority margin of -0.35 for paired read versus pre-contrast read while in Study A, Gadavist and gadoteridol did not.
- For the visualization endpoints contrast enhancement, border delineation, and internal morphology, the percentage of patients scoring higher for paired images compared to pre-contrast images ranged from 93% to 99% for Study A, and 95% to 97% for Study B. For both studies, the mean number of lesions detected on paired images exceeded that of the pre-contrast images; 37% for Study A and 24% for Study B. There were 29% and 11% of subjects in which the pre-contrast images detected more lesions for Study A and Study B, respectively.
- The percentage of patients whose average reader mean score changed by ≤ 0, up to 1, up to 2, and ≥ 2 scoring categories presented in Table 5 is shown in Table 7. The categorical improvement of (≤ 0) represents higher (< 0) or identical (= 0) scores for the pre-contrast read, the categories with scores > 0 represent the magnitude of improvement seen for the paired read.
- For both studies, the improvement of visualization endpoints in paired Gadavist images compared to pre-contrast images resulted in improved assessment of normal and abnormal CNS anatomy.
- Pediatric Patients
- Two studies in 44 pediatrics patients age younger than 2 years and 135 pediatric patients age 2 to less than18 years with CNS and non-CNS lesions supported extrapolation of adult CNS efficacy findings. For example, comparing pre vs paired pre- and post-contrast images, investigators selected the best of four descriptors under the heading, “Visualization of lesion-internal morphology (lesion characterization) or homogeneity of vessel enhancement” for 27/44 (62% = pre) vs 43/44 (98% = paired) MR images from patients age 0 to less than 2 years and 106/135 (78% = pre) vs 108/135 (80% = paired) MR images from patients age 2 to less than 18 years.
MRI of the Breast
- Patients with recently diagnosed breast cancer were enrolled in two identical clinical trials to evaluate the ability of Gadavist to assess the presence and extent of malignant breast disease prior to surgery. Patients underwent non-contrast breast MRI (BMR) prior to Gadavist (0.1 mmol/kg) breast MRI. BMR images and Gadavist BMR (combined contrast plus non-contrast) images were independently evaluated in each study by three readers blinded to clinical information. In separate reading sessions the BMR images and Gadavist BMR images were also interpreted together with X-ray mammography images (XRM).
- The studies evaluated 787 patients: Study 1 enrolled 390 women with an average age of 56 years, 74% were white, 25% Asian, 0.5% black, and 0.5% other; Study 2 enrolled 396 women and 1 man with an average age of 57 years, 71% were white, 24% Asian, 3% black, and 2% other.
- The readers assessed 5 regions per breast for the presence of malignancy using each reading modality. The readings were compared to an independent standard of truth (SoT) consisting of histopathology for all regions where excisions were made and tissue evaluated. XRM plus ultrasound was used for all other regions.
- The assessment of malignant disease was performed using a region based within-subject sensitivity. Sensitivity for each reading modality was defined as the mean of the percentage of malignant breast regions correctly interpreted for each subject. The within-subject sensitivity of Gadavist BMR was superior to that of BMR. The lower bound of the 95% Confidence Interval (CI) for the difference in within-subject sensitivity ranged from 19% to 42% for Study 1 and from 12% to 27% for Study 2. The within-subject sensitivity for Gadavist BMR and BMR as well as for Gadavist BMR plus XRM and BMR plus XRM is presented in Table 8.
- Specificity was defined as the percentage of non-malignant breasts correctly identified as non-malignant. The lower limit of the 95% confidence interval for specificity of Gadavist BMR was greater than 80% for 5 of 6 readers. (Table 9)
- Three additional readers in each study read XRM alone. For these readers over both studies, sensitivity ranged from 68% to 73% and specificity in non-malignant breasts ranged from 86% to 94%.
- In breasts with malignancy, a false positive detection rate was calculated as the percentage of subjects for which the readers assessed a region as malignant which could not be verified by SoT. The false positive detection rates for Gadavist BMR ranged from 39% to 53% (95% CI Upper Bounds ranged from 44% to 58%).
How Supplied
- Gadavist is a sterile, clear and colorless to pale yellow solution containing 604.72 mg gadobutrol per mL (equivalent to 1 mmol gadobutrol) per mL. Gadavist is supplied in the following sizes:
- Single-Dose Vials
- 2 mL single-dose vials, rubber stoppered in cartons of 3, Boxes of 15 (NDC 50419-325-37)
- 7.5 mL single-dose vials, rubber stoppered in cartons of 10, Boxes of 20 (NDC 50419-325-11)
- 10 mL single-dose vials, rubber stoppered, in cartons of 10, Boxes of 20 (NDC 50419-325-12)
- 15 mL single-dose vials, rubber stoppered, in cartons of 10, Boxes of 20 (NDC 50419-325-13)
- Single-Dose Pre-Filled Syringes
- 7.5 mL single-dose pre-filled disposable syringes, Boxes of 5 (NDC 50419-325-27)
- 10 mL single-dose pre-filled disposable syringes, Boxes of 5 (NDC 50419-325-28)
- 15 mL single-dose pre-filled disposable syringes, Boxes of 5 (NDC 50419-325-29)
- Storage and Handling
- Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F).
- Should freezing occur, Gadavist should be brought to room temperature before use. If allowed to stand at room temperature, Gadavist should return to a clear and colorless to pale yellow solution. Visually inspect Gadavist for particulate matter and discoloration prior to administration. Do not use the solution if it is discolored, if particulate matter is present or if the container appears damaged.
Storage
There is limited information regarding Gadobutrol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Gadobutrol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Gadobutrol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Nephrogenic Systemic Fibrosis
- Instruct patients to inform their physician if they:
- Have a history of kidney disease and/or liver disease, or
- Have recently received a GBCA
- GBCAs increase the risk of NSF among patients with impaired elimination of drugs. To counsel patients at risk of NSF:
- Describe the clinical manifestation of NSF
- Describe procedures to screen for the detection of renal impairment
- Instruct the patients to contact their physician if they develop signs or symptoms of NSF following Gadavist administration, such as burning, itching, swelling, scaling, hardening and tightening of the skin; red or dark patches on the skin; stiffness in joints with trouble moving, bending or straightening the arms, hands, legs or feet; pain in the hip bones or ribs; or muscle weakness.
Common Adverse Reactions
- Inform patients that they may experience:
General Precautions
- Instruct patients receiving Gadavist to inform their physician if they:
- Are pregnant or breastfeeding
- Have a history of allergic reaction to contrast media, bronchial asthma or allergic respiratory disorder.
Precautions with Alcohol
- Alcohol-Gadobutrol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- GADAVIST®[1]
Look-Alike Drug Names
There is limited information regarding Gadobutrol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Gadobutrol
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Gadobutrol |Label Name=Gadobutrol10.png
}}
{{#subobject:
|Label Page=Gadobutrol |Label Name=Gadobutrol11.png
}}










