Clindamycin hydrochloride (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Clindamycin hydrochloride (oral) is an antibiotic that is FDA approved for the treatment of empyema, anaerobic pneumonitis, and lung abscess; serious skin and soft tissue infections; septicemia; intra-abdominal infections such as peritonitis and intra-abdominal abscess. There is a Black Box Warning for this drug as shown here. Common adverse reactions include abdominal pain, pseudomembranous colitis, esophagitis, nausea, vomiting, and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Clindamycin is indicated in the treatment of serious infections caused by susceptible anaerobic bacteria.
- Clindamycin is also indicated in the treatment of serious infections due to susceptible strains of streptococci, pneumococci, and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgment of the physician, a penicillin is inappropriate. Because of the risk of colitis, before selecting clindamycin, the physician should consider the nature of the infection and the suitability of less toxic alternatives (e.g., erythromycin).
- Anaerobes: Serious respiratory tract infections such as empyema, anaerobic pneumonitis, and lung abscess; serious skin and soft tissue infections; septicemia; intra-abdominal infections such as peritonitis and intra-abdominal abscess (typically resulting from anaerobic organisms resident in the normal gastrointestinal tract); infections of the female pelvis and genital tract such as endometritis, non gonococcal tubo-ovarian abscess, pelvic cellulitis, and postsurgical vaginal cuff infection.
- Streptococci: Serious respiratory tract infections; serious skin and soft tissue infections.
- Staphylococci: Serious respiratory tract infections; serious skin and soft tissue infections.
- Pneumococci: Serious respiratory tract infections.
- Bacteriologic studies should be performed to determine the causative organisms and their susceptibility to clindamycin.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin hydrochloride and other antibacterial drugs, clindamycin hydrochloride should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage
- Adults: Serious infections – 150 to 300 mg every 6 hours. More severe infections – 300 to 450 mg every 6 hours. Pediatric Patients: Serious infections – 8 to 16 mg/kg/day (4 to 8 mg/lb/day) divided into three or four equal doses. More severe infections – 16 to 20 mg/kg/day (8 to 10 mg/lb/day) divided into three or four equal doses.
- To avoid the possibility of esophageal irritation, clindamycin hydrochloride capsules should be taken with a full glass of water.
- Serious infections due to anaerobic bacteria are usually treated with clindamycin injection. However, in clinically appropriate circumstances, the physician may elect to initiate treatment or continue treatment with clindamycin hydrochloride capsules.
- In cases of β-hemolytic streptococcal infections, treatment should continue for at least 10 days.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clindamycin hydrochloride (oral) in adult patients.
Non–Guideline-Supported Use
- Babesiosis
- Bacterial endocarditis; Prophylaxis
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- Clindamycin is indicated in the treatment of serious infections caused by susceptible anaerobic bacteria.
- Clindamycin is also indicated in the treatment of serious infections due to susceptible strains of streptococci, pneumococci, and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgment of the physician, a penicillin is inappropriate. Because of the risk of colitis, before selecting clindamycin, the physician should consider the nature of the infection and the suitability of less toxic alternatives (e.g., erythromycin).
- Anaerobes: Serious respiratory tract infections such as empyema, anaerobic pneumonitis, and lung abscess; serious skin and soft tissue infections; septicemia; intra-abdominal infections such as peritonitis and intra-abdominal abscess (typically resulting from anaerobic organisms resident in the normal gastrointestinal tract); infections of the female pelvis and genital tract such as endometritis, non gonococcal tubo-ovarian abscess, pelvic cellulitis, and postsurgical vaginal cuff infection.
- Streptococci: Serious respiratory tract infections; serious skin and soft tissue infections.
- Staphylococci: Serious respiratory tract infections; serious skin and soft tissue infections.
- Pneumococci: Serious respiratory tract infections.
- Bacteriologic studies should be performed to determine the causative organisms and their susceptibility to clindamycin.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin hydrochloride and other antibacterial drugs, clindamycin hydrochloride should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy
Dosage
- Serious infections - 8 to 16 mg/kg/day (4 to 8 mg/lb/day) divided into three or four equal doses. More severe infections - 16 to 20 mg/kg/day (8 to 10 mg/lb/day) divided into three or four equal doses
- To avoid the possibility of esophageal irritation, clindamycin hydrochloride capsules should be taken with a full glass of water.
- Serious infections due to anaerobic bacteria are usually treated with clindamycin injection. However, in clinically appropriate circumstances, the physician may elect to initiate treatment or continue treatment with clindamycin hydrochloride capsules.
- In cases of β-hemolytic streptococcal infections, treatment should continue for at least 10 days.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clindamycin hydrochloride (oral) in adult patients.
Non–Guideline-Supported Use
Babesiosis
Dosage
Babesiosis: 7 to 10 mg/kg ORALLY every 6 to 8 hours (max 600 mg/dose) plus quinine 8 mg/kg orally every 8 hours (MAX 650 mg/dose) for 7 to 10 days
Bacterial endocarditis; Prophylaxis
Dosage
Bacterial endocarditis; Prophylaxis: (high-risk patients; dental, respiratory, or infected skin/skin structure or musculoskeletal tissue procedures)
Dosage
20 mg/kg orally 30 to 60 minutes prior to procedure.
Contraindications
Clindamycin hydrochloride is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
|
There is limited information regarding Clindamycin hydrochloride (oral) Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
The following reactions have been reported with the use of clindamycin.
Gastrointestinal: Abdominal pain, pseudomembranous colitis, esophagitis, nausea, vomiting, and diarrhea . The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment . Esophageal ulcer has been reported.
Hypersensitivity Reactions: Generalized mild to moderate morbilliform-like (maculopapular) skin rashes are the most frequently reported adverse reactions. Vesiculobullous rashes, as well as urticaria, have been observed during drug therapy. Severe skin reactions such as toxic epidermal necrolysis, some with fatal outcome, have been reported . Cases of acute generalized exanthematous pustulosis (AGEP), erythema multiforme, some resembling Stevens-Johnson syndrome, and anaphylactoid reactions have also been reported.
Skin and Mucous Membranes: Pruritus, vaginitis, and rare instances of exfoliative dermatitis have been reported.
Liver: Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Renal: Although no direct relationship of clindamycin to renal damage has been established, renal dysfunction as evidenced by azotemia , oliguria, and/or proteinuria has been observed.
Hematopoietic: Transient neutropenia (leukopenia) and eosinophilia have been reported. Reports of agranulocytosis and thrombocytopenia have been made. No direct etiologic relationship to concurrent clindamycin therapy could be made in any of the foregoing.
Immune System: Drug reaction with eosinophilia and systemic symptoms (DRESS) cases have been reported.
Musculoskeletal: Cases of polyarthritis have been reported.
Postmarketing Experience
There is limited information regarding Clindamycin hydrochloride (oral) Postmarketing Experience in the drug label.
Drug Interactions
- Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
- Antagonism has been demonstrated between clindamycin and erythromycin in vitro. Because of possible clinical significance, these two drugs should not be administered concurrently.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B Teratogenic Effects
- In clinical trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters, has not been associated with an increased frequency of congenital abnormalities.
- Clindamycin should be used during the first trimester of pregnancy only if clearly needed. There are no adequate and well-controlled studies in pregnant women during the first trimester of pregnancy. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed.
- Reproduction studies performed in rats and mice using oral doses of clindamycin up to 600 mg/kg/day (3.2 and 1.6 times the highest recommended adult human dose based on mg/m2, respectively) or subcutaneous doses of clindamycin up to 250 mg/kg/day (1.3 and 0.7 times the highest recommended adult human dose based on mg/m2, respectively) revealed no evidence of teratogenicity.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clindamycin hydrochloride (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Clindamycin hydrochloride (oral) during labor and delivery.
Nursing Mothers
- Clindamycin has been reported to appear in breast milk in the range of 0.7 to 3.8 mcg/mL. Because of the potential for serious adverse reactions in nursing infants, clindamycin should not be taken by nursing mothers.
Pediatric Use
- When clindamycin hydrochloride is administered to the pediatric population (birth to 16 years), appropriate monitoring of organ system functions is desirable.
Geriatic Use
- Clinical studies of clindamycin did not include sufficient numbers of patients age 65 and over to determine whether they respond differently from younger patients. However, other reported clinical experience indicates that antibiotic-associated colitis and diarrhea (due to Clostridium difficile) seen in association with most antibiotics occur more frequently in the elderly (> 60 years) and may be more severe. These patients should be carefully monitored for the development of diarrhea.
- Pharmacokinetic studies with clindamycin have shown no clinically important differences between young and elderly subjects with normal hepatic function and normal (age-adjusted) renal function after oral or intravenous administration.
Gender
There is no FDA guidance on the use of Clindamycin hydrochloride (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clindamycin hydrochloride (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Clindamycin hydrochloride (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Clindamycin hydrochloride (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clindamycin hydrochloride (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clindamycin hydrochloride (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- If significant diarrhea occurs during therapy, this antibiotic should be discontinued.
IV Compatibility
- There is limited information regarding IV Compatibility of Clindamycin hydrochloride (oral) in the drug label.
Overdosage
- Significant mortality was observed in mice at an intravenous dose of 855 mg/kg and in rats at an oral or subcutaneous dose of approximately 2618 mg/kg. In the mice, convulsions and depression were observed.
- Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum.
Pharmacology
Mechanism of Action
- Clindamycin has a primarily bacteriostatic effect. It is a bacterial protein synthesis inhibitor by inhibiting ribosomal translocation,[1] in a similar way to macrolides. It does so by binding to the 50S rRNA of the large bacterial ribosome subunit.[2]
- The structures of the complexes between several antibiotics (including clindamycin) and a Deinococcus radiodurans ribosome have been solved by X-ray crystallography by a team from the Max Planck Working Groups for Structural Molecular Biology, and published in the journal Nature.[3]
Structure
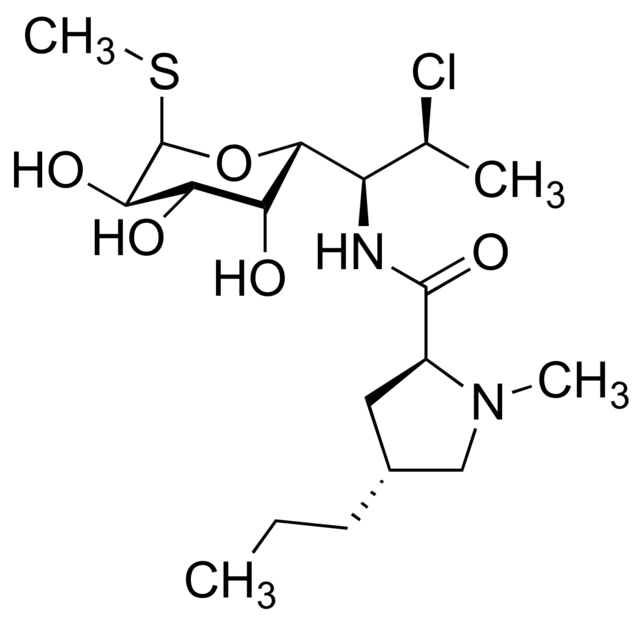
- Clindamycin hydrochloride is the hydrated hydrochloride salt of clindamycin. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent compound lincomycin.
- CLEOCIN HCl Capsules contain clindamycin hydrochloride equivalent to 75 mg, 150 mg, or 300 mg of clindamycin.
- Inactive ingredients: 75 mg – corn starch, FD&C blue no. 1, FD&C yellow no. 5, gelatin, lactose, magnesium stearate, and talc; 150 mg – corn starch, FD&C blue no. 1, FD&C yellow no. 5, gelatin, lactose, magnesium stearate, talc and titanium dioxide; 300 mg – corn starch, FD&C blue no. 1, gelatin, lactose, magnesium stearate, talc, and titanium dioxide.
The structural formula is represented below:

- The chemical name for clindamycin hydrochloride is Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside monohydrochloride.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Clindamycin hydrochloride (oral) in the drug label.
Pharmacokinetics
Absorption
Serum level studies with a 150 mg oral dose of clindamycin hydrochloride in 24 normal adult volunteers showed that clindamycin was rapidly absorbed after oral administration. An average peak serum level of 2.50 mcg/mL was reached in 45 minutes; serum levels averaged 1.51 mcg/mL at 3 hours and 0.70 mcg/mL at 6 hours. Absorption of an oral dose is virtually complete (90%), and the concomitant administration of food does not appreciably modify the serum concentrations; serum levels have been uniform and predictable from person to person and dose to dose. Serum level studies following multiple doses of CLEOCIN HCl for up to 14 days show no evidence of accumulation or altered metabolism of drug. Doses of up to 2 grams of clindamycin per day for 14 days have been well tolerated by healthy volunteers, except that the incidence of gastrointestinal side effects is greater with the higher doses.
Distribution
Concentrations of clindamycin in the serum increased linearly with increased dose. Serum levels exceed the MIC (minimum inhibitory concentration) for most indicated organisms for at least six hours following administration of the usually recommended doses. Clindamycin is widely distributed in body fluids and tissues (including bones). No significant levels of clindamycin are attained in the cerebrospinal fluid, even in the presence of inflamed meninges.
Excretion
The average biological half-life is 2.4 hours. Approximately 10% of the bioactivity is excreted in the urine and 3.6% in the feces; the remainder is excreted as bioinactive metabolites.
Special Populations
Renal Impairment
Serum half-life of clindamycin is increased slightly in patients with markedly reduced renal function. Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum.
Use in Elderly
Pharmacokinetic studies in elderly volunteers (61–79 years) and younger adults (18–39 years) indicate that age alone does not alter clindamycin pharmacokinetics (clearance, elimination half-life, volume of distribution, and area under the serum concentration-time curve) after IV administration of clindamycin phosphate. After oral administration of clindamycin hydrochloride, elimination half-life is increased to approximately 4.0 hours (range 3.4–5.1 h) in the elderly compared to 3.2 hours (range 2.1 – 4.2 h) in younger adults. The extent of absorption, however, is not different between age groups and no dosage alteration is necessary for the elderly with normal hepatic function and normal (age-adjusted) renal function.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential. Genotoxicity tests performed included a rat micronucleus test and an Ames Salmonella reversion test. Both tests were negative.
- Fertility studies in rats treated orally with up to 300 mg/kg/day (approximately 1.6 times the highest recommended adult human dose based on mg/m2) revealed no effects on fertility or mating ability.
Clinical Studies
There is limited information regarding Clinical Studies of Clindamycin hydrochloride (oral) in the drug label.
How Supplied
Clindamycin hydrochloride Capsules are available in the following strengths, colors and sizes:

Storage
Store at controlled room temperature 20° to 25° C (68° to 77° F)
Images
Drug Images
{{#ask: Page Name::Clindamycin hydrochloride (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Clindamycin hydrochloride (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be counseled that antibacterial drugs, including clindamycin hydrochloride capsules, USP, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When clindamycin hydrochloride capsules, USP are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by clindamycin hydrochloride capsules, USP or other antibacterial drugs in the future.
- Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Precautions with Alcohol
Alcohol-Clindamycin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CLINDAMYCIN HYDROCHLORIDE
Look-Alike Drug Names
There is limited information regarding Clindamycin hydrochloride (oral) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Clindamycin University of Michigan. Retrieved July 31, 2009
- ↑ "Lincosamides, Oxazolidinones, and Streptogramins". Merck Manual of Diagnosis and Therapy. Merck & Co. November 2005. Retrieved 2007-12-01.
- ↑ Schlünzen F, Zarivach R, Harms J; et al. (2001). "Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria". Nature. 413 (6858): 814–21. doi:10.1038/35101544. PMID 11677599.