Cetirizine ophthalmic solution (Zerviate)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand, Anmol Pitliya, M.B.B.S. M.D.[2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cetirizine ophthalmic solution (Zerviate) is a histamine-1 (H1) receptor antagonist that is FDA approved for the treatment of ocular itching associated with allergic conjunctivitis. Common adverse reactions include ocular hyperemia, instillation site pain, and visual acuity reduced.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Cetirizine ophthalmic solution 0.24% is indicated for the treatment of ocular itching associated with allergic conjunctivitis.
Dosage and Administration
- The recommended dosage of cetirizine ophthalmic solution is to instill one drop in each affected eye twice daily (approximately 8 hours apart).
Dosage Forms and Strengths
- Cetirizine ophthalmic solution, 0.24% is a sterile, buffered, clear, colorless aqueous solution containing cetirizine 0.24% (equivalent to cetirizine hydrochloride 0.29%).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding ceturuzube ophthalmic solution Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding ceturuzube ophthalmic solution Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indication
- Cetirizine ophthalmic solution 0.24% is indicated for the treatment of ocular itching associated with allergic conjunctivitis.
Dosage and Administration
- The recommended dosage of cetirizine ophthalmic solution is to instill one drop in each affected eye twice daily (approximately 8 hours apart).
Dosage Forms and Strengths
- Cetirizine ophthalmic solution, 0.24% is a sterile, buffered, clear, colorless aqueous solution containing cetirizine 0.24% (equivalent to cetirizine hydrochloride 0.29%).
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding ceturuzube ophthalmic solution Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding ceturuzube ophthalmic solution Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None.
Warnings
Contamination of Tip and Solution
- As with any eye drop, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle to prevent contaminating the tip and solution. Keep the bottle closed when not in use.
Contact Lens Wear
- Patients should be advised not to wear a contact lens if their eye is red.
- Cetirizine ophthalmic solution should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of cetirizine ophthalmic solution. The preservative in cetirizine ophthalmic solution, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of cetirizine ophthalmic solution.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trial of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates in practice.
- In seven clinical trials, patients with allergic conjunctivitis or those at a risk of developing allergic conjunctivitis received one drop of either cetirizine (N=511) or vehicle (N=329) in one or both eyes. The most commonly reported adverse reactions occurred in approximately 1–7% of patients treated with either cetirizine ophthalmic solution or vehicle. These reactions were ocular hyperemia, instillation site pain, and visual acuity reduced.
Postmarketing Experience
There is limited information regarding Cetirizine ophthalmic solution (Zerviate) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Cetirizine ophthalmic solution (Zerviate) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Risk Summary
- There were no adequate or well-controlled studies with cetirizine ophthalmic solution 0.24% in pregnant women. Cetirizine should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Data (Animal)
- Cetirizine was not teratogenic in mice, rats, or rabbits at oral doses up to 96, 225, and 135 mg/kg, respectively (approximately 1300, 4930, and 7400 times the maximum recommended human ophthalmic dose (MRHOD), on a mg/m2 basis).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cetirizine ophthalmic solution (Zerviate) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cetirizine ophthalmic solution (Zerviate) during labor and delivery.
Nursing Mothers
Risk Summary
- Cetirizine has been reported to be excreted in human breast milk following oral administration. Multiple doses of oral dose cetirizine (10 mg tablets once daily for 10 days) resulted in systemic levels (Mean Cmax = 311 ng/mL) that were 100 times higher than the observed human exposure (Mean Cmax = 3.1 ng/mL) following twice daily administration of cetirizine ophthalmic solution 0.24% to both eyes for one week. Comparable bioavailability has been found between the tablet and syrup dosage forms. However, it is not known whether the systemic absorption resulting from topical ocular administration of cetirizine ophthalmic solution could produce detectable quantities in human breast milk.
- There is no adequate information regarding the effects of cetirizine on breastfed infants, or the effects on milk production to inform risk of cetirizine ophthalmic solution to an infant during lactation. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for cetirizine ophthalmic solution and any potential adverse effects on the breastfed child from cetirizine ophthalmic solution.
Pediatric Use
- The safety and effectiveness of cetirizine ophthalmic solution has been established in pediatric patients two years of age and older. Use of cetirizine ophthalmic solution in these pediatric patients is supported by evidence from adequate and well-controlled studies of cetirizine ophthalmic solution in pediatric and adult patients.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Cetirizine ophthalmic solution (Zerviate) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cetirizine ophthalmic solution (Zerviate) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cetirizine ophthalmic solution (Zerviate) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cetirizine ophthalmic solution (Zerviate) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cetirizine ophthalmic solution (Zerviate) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cetirizine ophthalmic solution (Zerviate) in patients who are immunocompromised.
Administration and Monitoring
Administration
- The recommended dosage of cetirizine ophthalmic solution is to instill one drop in each affected eye twice daily (approximately 8 hours apart).
Monitoring
- Improvement in rhinitis symptoms.
- Urticaria: improvement in itching and or/ hives.
- CNS effects.
IV Compatibility
There is limited information regarding the compatibility of Cetirizine ophthalmic solution (Zerviate) and IV administrations.
Overdosage
There is limited information regarding Cetirizine ophthalmic solution (Zerviate) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
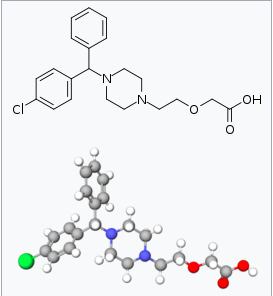
| |
| Template:Px | |
Cetirizine ophthalmic solution (Zerviate)
| |
| Systematic (IUPAC) name | |
| (±)-[2-[4-[(4-chlorophenyl)phenylmethyl]-1- piperazinyl]ethoxy]acetic acid | |
| Identifiers | |
| CAS number | |
| ATC code | R06 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 388.89 g/mol |
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed (>70%) |
| Protein binding | 88–96% |
| Metabolism | Minimal (non-cytochrome P450-mediated) |
| Half life | Mean: 8.3 hours |
| Excretion | Urine: 70–85%, Feces: 10–13% |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
B(US) |
| Legal status | |
| Routes | By mouth |
Mechanism of Action
- Cetirizine ophthalmic solution, an antihistamine, is a histamine-1 (H1) receptor antagonist. Its effects are mediated via selective inhibition of H1 histamine receptors. The antihistaminic activity of cetirizine has been documented in a variety of animal and human models. In vivo and ex vivo animal models have shown negligible anticholinergic and antiserotonergic activity. In vitro receptor binding studies have shown no measurable affinity for other than H1 receptors.
Structure

Pharmacodynamics
There is limited information regarding Cetirizine ophthalmic solution (Zerviate) Pharmacodynamics in the drug label.
Pharmacokinetics
- In healthy subjects, bilateral topical ocular dosing of one drop of cetirizine ophthalmic solution 0.24% resulted in a mean cetirizine plasma Cmax of 1.7 ng/mL following a single dose and 3.1 ng/mL after twice-daily dosing for one week. The observed mean terminal half-life of cetirizine was 8.6 hours following a single dose and 8.2 hours after twice-daily dosing of cetirizine ophthalmic solution for one week.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
- In a 2-year carcinogenicity study in rats, orally administered cetirizine was not carcinogenic at dietary doses up to 20 mg/kg (approximately 550 times the MRHOD, on a mg/m2 basis). In a 2-year carcinogenicity study in mice, cetirizine caused an increased incidence of benign liver tumors in males at a dietary dose of 16 mg/kg (approximately 220 times the MRHOD, on a mg/m2 basis). No increase in the incidence of liver tumors was observed in mice at a dietary dose of 4 mg/kg (approximately 55 times the MRHOD, on a mg/m2 basis). The clinical significance of these findings during long-term use of cetirizine is not known.
Mutagenesis
- Cetirizine was not mutagenic in the Ames test or in an in vivo micronucleus test in rats. Cetirizine was not clastogenic in the human lymphocyte assay or the mouse lymphoma assay.
Impairment of Fertility
- In a fertility and general reproductive performance study in mice, cetirizine did not impair fertility at an oral dose of 64 mg/kg (approximately 875 times the MRHOD on a mg/m2 basis).
Clinical Studies
- The efficacy of cetirizine ophthalmic solution was established in three randomized, double-masked, placebo-controlled, conjunctival allergen challenge (CAC) clinical trials in patients with a history of allergic conjunctivitis. Onset and duration of action were evaluated in two of these trials in which patients were randomized to receive cetirizine ophthalmic solution or vehicle ophthalmic solutions. Patients were evaluated with an ocular itching severity score ranging from 0 (no itching) to 4 (incapacitating itch) at several time points after CAC administration. Table 1 displays data from the mean ocular itching severity scores after ocular administration of an antigen using the CAC model. A one unit difference compared to vehicle is considered a clinically meaningful change in the ocular itching severity score.
- Patients treated with cetirizine ophthalmic solution demonstrated statistically and clinically significantly less ocular itching compared to vehicle at 15 minutes and 8 hours after treatment.

How Supplied
- Cetirizine ophthalmic solution is a sterile, buffered, clear, colorless aqueous solution containing cetirizine 0.24% (equivalent to cetirizine hydrochloride 0.29%) supplied in a white low-density polyethylene multi-dose ophthalmic bottle with a low-density polyethylene dropper tip and a white polypropylene cap. Cetirizine ophthalmic solution is supplied in a 7.5 mL bottle that contains 5 mL and 10 mL bottle that contains 7.5 mL cetirizine ophthalmic solution, 2.40 mg [equivalent to 2.85 mg cetirizine hydrochloride in one mL solution].
- 5 mL fill in a 7.5 mL bottle NDC XXXXX-XXXX-5
- 7.5 mL fill in a 10 mL bottle NDC XXXXX-XXXX-7
Storage
- Store at 15°C to 25°C (59°F to 77°F).
Images
Drug Images
{{#ask: Page Name::Cetirizine ophthalmic solution (Zerviate) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cetirizine ophthalmic solution (Zerviate) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Risk of Contamination: Advise patients not to touch dropper tip to eyelids or surrounding areas, as this may contaminate the dropper tip and ophthalmic solution. Advise patients to keep the bottle closed when not in use.
- Concomitant Use of Contact Lenses: Advise patients not to wear contact lenses if their eyes are red. Advise patients that cetirizine ophthalmic solution should not be used to treat contact lens related irritation. Advise patients to remove contact lenses prior to instillation of cetirizine ophthalmic solution. The preservative in cetirizine ophthalmic solution solution, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted ten minutes following administration of cetirizine ophthalmic solution.
Precautions with Alcohol
Alcohol-Cetirizine ophthalmic solution (Zerviate) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Zerviate
Look-Alike Drug Names
There is limited information regarding Cetirizine ophthalmic solution (Zerviate) Look-Alike Drug Names in the drug label.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.