Cefazolin sodium indications and usage
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Indications and Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefazolin and other antibacterial drugs, cefazolin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Cefazolin for Injection is indicated for the treatment of the following infections when caused by susceptible bacteria.
Respiratory Tract Infections
Respiratory tract infections due to Streptococcus pneumoniae, Staphylococcus aureus and Streptococcus pyogenes.
Injectable benzathine penicillin is considered the drug of choice in treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever.
Cefazolin is effective in the eradication of streptococci from the nasopharynx; however, data establishing the efficacy of cefazolin in the subsequent prevention of rheumatic fever are not available.
Urinary Tract Infections
Urinary tract infections due to Escherichia coli and Proteus mirabilis.
Skin and Skin Structure Infections
Skin and skin structure infections due to S. aureus, S. pyogenes, and Streptococcus agalactiae.
Biliary Tract Infections
Biliary infections due to E. coli, various isolates ofstreptococci, P. mirabilis, and S. aureus.
Bone and Joint Infections
Bone and joint infections due to S. aureus.
Genital Infections
Genital infections due to E. coli and P. mirabilis.
Septicemia
Septicemia due to S. pneumoniae, S. aureus, P. mirabilis, and E. coli.
Endocarditis
Endocarditis due to S. aureus and S. pyogenes.
Perioperative Prophylaxis
The prophylactic administration of cefazolin preoperatively, intraoperatively, and postoperatively may reduce the incidence of certain postoperative infections in patients undergoing surgical procedures which are classified as contaminated or potentially contaminated (e.g., vaginal hysterectomy, and cholecystectomy in high-risk patients such as those older than 70 years, with acute cholecystitis, obstructive jaundice, or common duct bile stones).
The perioperative use of cefazolin may also be effective in surgical patients in whom infection at the operative site would present a serious risk (e.g., during open-heart surgery and prosthetic arthroplasty).
If there are signs of infection, specimens for cultures should be obtained for the identification of the causative organism so that appropriate therapy may be instituted.
Dosage Adjustment for Patients With Reduced Renal Function
Cefazolin may be used in patients with reduced renal function with the following dosage adjustments: Patients with a creatinine clearance of 55 mL/min. or greater or a serum creatinine of 1.5 mg % or less can be given full doses. Patients with creatinine clearance rates of 35 to 54 mL/min. or serum creatinine of 1.6 to 3 mg % can also be given full doses but dosage should be restricted to at least 8 hour intervals. Patients with creatinine clearance rates of 11 to 34 mL/min. or serum creatinine of 3.1 to 4.5 mg % should be given ½ the usual dose every 12 hours. Patients with creatinine clearance rates of 10 mL/min. or less or serum creatinine of 4.6 mg % or greater should be given ½ the usual dose every 18 to 24 hours. All reduced dosage recommendations apply after an initial loading dose appropriate to the severity of the infection. Patients undergoing peritoneal dialysis: See CLINICAL PHARMACOLOGY.
Pediatric Dosage
In pediatric patients, a total daily dosage of 25 to 50 mg/kg (approximately 10 to 20 mg/lb) of body weight, divided into 3 or 4 equal doses, is effective for most mild to moderately severe infections. Total daily dosage may be increased to 100 mg/kg (45 mg/lb) of body weight for severe infections. Since safety for use in premature infants and in neonates has not been established, the use of cefazolin in these patients is not recommended.
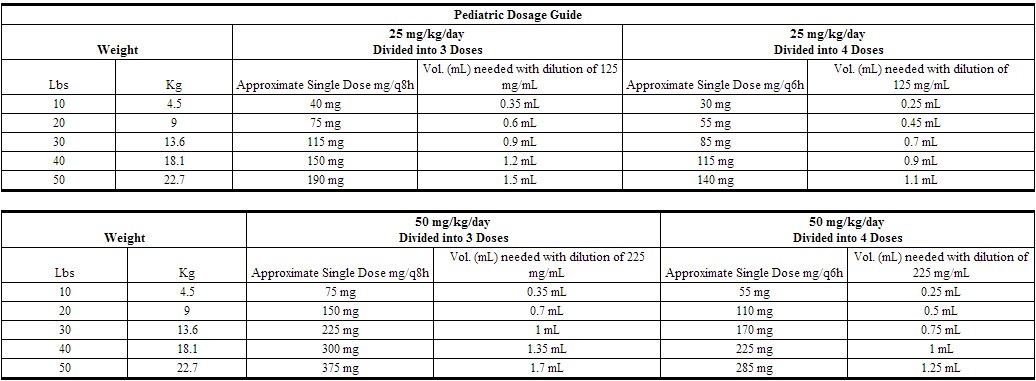 |
In pediatric patients with mild to moderate renal impairment (creatinine clearance of 70 to 40 mL/min.), 60 percent of the normal daily dose given in equally divided doses every 12 hours should be sufficient. In patients with moderate impairment (creatinine clearance of 40 to 20 mL/min.), 25 percent of the normal daily dose given in equally divided doses every 12 hours should be adequate. Pediatric patients with severe renal impairment (creatinine clearance of 20 to 5 mL/min.) may be given 10 percent of the normal daily dose every 24 hours. All dosage recommendations apply after an initial loading dose.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/50461slr139_ancef_lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.