Carfilzomib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]; Sree Teja Yelamanchili, MBBS [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Carfilzomib is a proteasome inhibitor that is FDA approved for the treatment of multiple myeloma. Common adverse reactions include fatigue, anemia, nausea, thrombocytopenia, dyspnea, diarrhea, and pyrexia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Multiple Myeloma
- Carfilzomib is indicated for the treatment of patients with multiple myeloma who have received at least two prior therapies including bortezomib and an immunomodulatory agent and have demonstrated disease progression on or within 60 days of completion of the last therapy. Approval is based on response rate.
- Dosing Guidelines
- Carfilzomib is administered intravenously over 2 to 10 minutes, on two consecutive days, each week for three weeks (Days 1, 2, 8, 9, 15, and 16), followed by a 12-day rest period (Days 17 to 28). Each 28-day period is considered one treatment cycle (Table 1).
- In Cycle 1, Carfilzomib is administered at a dose of 20 mg/m2. If tolerated in Cycle 1, the dose should be escalated to 27 mg/m2 beginning in Cycle 2 and continued at 27 mg/m2 in subsequent cycles. Treatment may be continued until disease progression or until unacceptable toxicity occurs.
- The dose is calculated using the patient’s actual body surface area at baseline. Patients with a body surface area greater than 2.2 m2 should receive a dose based upon a body surface are of 2.2 m2. Dose adjustments do not need to be made for weight changes of less than or equal to 20%.
- Hydration and Fluid Monitoring
- Hydrate patients to reduce the risk of renal toxicity and of tumor lysis syndrome (TLS) with Carfilzomib treatment. Maintain adequate fluid volume status throughout treatment and monitor blood chemistries closely. Prior to each dose in Cycle 1, give 250 mL to 500 mL of intravenous normal saline or other appropriate intravenous fluid. Give an additional 250 mL to 500 mL of intravenous fluids as needed following Carfilzomib administration. Continue intravenous hydration, as needed, in subsequent cycles. Also monitor patients during this period for fluid overload.
- Dexamethasone Premedication
- Pre-medicate with dexamethasone 4 mg orally or intravenously prior to all doses of Carfilzomib during Cycle 1 and prior to all Carfilzomib doses during the first cycle of dose escalation to 27 mg/m2 to reduce the incidence and severity of infusion reactions. Reinstate dexamethasone premedication (4 mg orally or intravenously) if these symptoms develop or reappear during subsequent cycles.
- Dose Modifications Based on Toxicities
- Recommended actions and dose modifications are presented in Table 2.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carfilzomib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carfilzomib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Carfilzomib in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carfilzomib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carfilzomib in pediatric patients.
Contraindications
- None.
Warnings
Precautions
- Death due to cardiac arrest has occurred within a day of Carfilzomib administration. New onset or worsening of pre-existing congestive heart failure with decreased left ventricular function or myocardial ischemia have occurred following administration of Carfilzomib. Cardiac failure events (e.g., cardiac failure congestive, pulmonary edema, ejection fraction decreased) were reported in 7% of patients. Monitor for cardiac complications and manage promptly. Withhold Carfilzomib for Grade 3 or 4 cardiac events until recovery and consider whether to restart Carfilzomib based on a benefit/risk assessment. Patients with New York Heart Association Class III and IV heart failure, myocardial infarction in the preceding 6 months, and conduction abnormalities uncontrolled by medications were not eligible for the clinical trials. These patients may be at greater risk for cardiac complications.
- Pulmonary Hypertension
- Pulmonary arterial hypertension (PAH) was reported in 2% of patients treated with Carfilzomib and was Grade 3 or greater in less than 1% of patients. Evaluate with cardiac imaging and/or other tests as indicated. Withhold Carfilzomib for pulmonary hypertension until resolved or returned to baseline and consider whether to restart Carfilzomib based on a benefit/risk assessment.
- Pulmonary Complications
- Infusion Reactions
- Infusion reactions were characterized by a spectrum of systemic symptoms including fever, chills, arthralgia, myalgia, facial flushing, facial edema, vomiting, weakness, shortness of breath, hypotension, syncope, chest tightness, or angina. These reactions can occur immediately following or up to 24 hours after administration of Carfilzomib. Administer dexamethasone prior to Carfilzomib to reduce the incidence and severity of reactions. Inform patients of the risk and symptoms and to contact physician if symptoms of an infusion reaction occur.
- Tumor Lysis Syndrome
- Tumor lysis syndrome (TLS) occurred following Carfilzomib administration in < 1% of patients. Patients with multiple myeloma and a high tumor burden should be considered to be at greater risk for TLS. Prior to receiving Carfilzomib, ensure that patients are well hydrated. Monitor for evidence of TLS during treatment, and manage promptly. Interrupt Carfilzomib until TLS is resolved.
- Thrombocytopenia
- Carfilzomib causes thrombocytopenia with platelet nadirs occurring around Day 8 of each 28-day cycle and recovery to baseline by the start of the next 28-day cycle. In patients with multiple myeloma, 36% of patients experienced thrombocytopenia, including Grade 4 in 10%. Thrombocytopenia following Carfilzomib administration resulted in a dose reduction in 1% of patients and discontinuation of treatment with Carfilzomib in < 1% of patients. Monitor platelet counts frequently during treatment with Carfilzomib. Reduce or interrupt dose as clinically indicated.
- Hepatic Toxicity and Hepatic Failure
- Cases of hepatic failure, including fatal cases, have been reported (< 1%). Carfilzomib can cause elevations of serum transaminases and bilirubin. Withhold Carfilzomib in patients experiencing Grade 3 or greater elevations of transaminases, bilirubin, or other liver abnormalities until resolved or returned to baseline. After resolution, consider if restarting Carfilzomib is appropriate. Monitor liver enzymes frequently.
- Embryo-fetal Toxicity
- Carfilzomib can cause fetal harm when administered to a pregnant woman based on its mechanism of action and findings in animals. There are no adequate and well-controlled studies in pregnant women using Carfilzomib. Carfilzomib caused embryo-fetal toxicity in pregnant rabbits at doses that were lower than in patients receiving the recommended dose.
- Females of reproductive potential should be advised to avoid becoming pregnant while being treated with Carfilzomib. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug, and may not reflect the rates observed in medical practice.
- A total of 526 patients with relapsed and/or refractory multiple myeloma received Carfilzomib as monotherapy or with pre-dose dexamethasone. Patients received a median of four treatment cycles with a median cumulative Carfilzomib dose of 993.4 mg.
- Deaths due to all causes within 30 days of the last dose of Carfilzomib occurred in 37/526 (7%) of patients. Deaths not attributed to disease progression were cardiac in 5 patients (acute coronary syndrome, cardiac arrest, cardiac disorder), end-organ failure in 4 patients (multi-organ failure, hepatic failure, renal failure), infection in 4 patients (sepsis, pneumonia, respiratory tract bacterial infection), dyspnea and intracranial hemorrhage in 1 patient each, and 1 patient found dead of unknown causes.
- Serious adverse reactions were reported in 45% patients. The most common serious adverse reactions were pneumonia (10%), acute renal failure (4%), pyrexia (3%), and congestive heart failure (3%). Adverse reactions leading to discontinuation of Carfilzomib occurred in 15% of patients and included congestive heart failure (2%), cardiac arrest, dyspnea, increased blood creatinine, and acute renal failure (1% each).
- Adverse reactions occurring at a rate of 10% or greater are presented in Table 4.
- Renal Events
- The most common renal adverse reactions were increase in blood creatinine (24%) and renal failure (9%), which were mostly Grade 1 or Grade 2 in severity. Grade 3 renal adverse reactions occurred in 6% of patients and Grade 4 events occurred in 1%. Discontinuations due to increased blood creatinine and acute renal failure were 1% each. In one patient, death occurred with concurrent sepsis and worsening renal function.
- Peripheral Neuropathy
- Peripheral neuropathy (including all events of peripheral sensory neuropathy and peripheral motor neuropathy) occurred in 14% of patients enrolled in clinical trials. Grade 3 peripheral neuropathy occurred in 1% of patients. Serious peripheral neuropathy events occurred in < 1% of patients, which resulted in dose reduction in < 1% and treatment discontinuation in < 1%. Withhold or discontinue treatment as recommended.
- Herpes Virus Infection
- Herpes zoster reactivation was reported in 2% of patients. Consider antiviral prophylaxis for patients who have a history of herpes zoster infection.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Carfilzomib in the drug label.
Drug Interactions
- Carfilzomib is primarily metabolized via peptidase and epoxide hydrolase activities, and as a result, the pharmacokinetic profile of carfilzomib is unlikely to be affected by concomitant administration of cytochrome P450 inhibitors and inducers. Carfilzomib is not expected to influence exposure of other drugs.
Use in Specific Populations
Pregnancy
- Pregnancy Category D
- Females of reproductive potential should be advised to avoid becoming pregnant while being treated with Carfilzomib. Based on its mechanism of action and findings in animals, Carfilzomib can cause fetal harm when administered to a pregnant woman. Carfilzomib caused embryo-fetal toxicity in pregnant rabbits at doses that were lower than in patients receiving the recommended dose. If Carfilzomib is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Carfilzomib was administered intravenously to pregnant rats and rabbits during the period of organogenesis at doses of 0.5, 1, and 2 mg/kg/day in rats and 0.2, 0.4, and 0.8 mg/kg/day in rabbits. Carfilzomib was not teratogenic at any dose tested. In rabbits, there was an increase in pre-implantation loss at ≥ 0.4 mg/kg/day and an increase in early resorptions and post-implantation loss and a decrease in fetal weight at the maternally toxic dose of 0.8 mg/kg/day. The doses of 0.4 and 0.8 mg/kg/day in rabbits are approximately 20% and 40%, respectively, of the recommended dose in humans of 27 mg/m2 based on body surface area.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Carfilzomib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Carfilzomib during labor and delivery.
Nursing Mothers
- It is not known whether Carfilzomib is excreted in human milk. Since many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Carfilzomib, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of Carfilzomib in pediatric patients have not been established.
Geriatic Use
- In studies of Carfilzomib there were no clinically significant differences observed in safety and efficacy between patients less than 65 years of age and patients 65 years of age and older.
Gender
There is no FDA guidance on the use of Carfilzomib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Carfilzomib with respect to specific racial populations.
Renal Impairment
- The pharmacokinetics and safety of Carfilzomib were evaluated in a Phase 2 trial in patients with normal renal function and those with mild, moderate, and severe renal impairment and patients on chronic dialysis. On average, patients were treated for 5.5 cycles using Carfilzomib doses of 15 mg/m2 on Cycle 1, 20 mg/m2 on Cycle 2, and 27 mg/m2 on Cycles 3 and beyond. The pharmacokinetics and safety of Carfilzomib were not influenced by the degree of baseline renal impairment, including the patients on dialysis. Since dialysis clearance of Carfilzomib concentrations has not been studied, the drug should be administered after the dialysis procedure.
Hepatic Impairment
- The safety, efficacy and pharmacokinetics of Carfilzomib have not been evaluated in patients with baseline hepatic impairment. Patients with the following laboratory values were excluded from the Carfilzomib clinical trials: ALT/AST ≥ 3 × upper limit of normal (ULN) and bilirubin ≥ 2 × ULN.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Carfilzomib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Carfilzomib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Carfilzomib in the drug label.
IV Compatibility
Overdosage
Chronic Overdose
There is limited information regarding Chronic Overdose of Carfilzomib in the drug label.
Pharmacology
Mechanism of Action
- Carfilzomib is a tetrapeptide epoxyketone proteasome inhibitor that irreversibly binds to the N-terminal threonine-containing active sites of the 20S proteasome, the proteolytic core particle within the 26S proteasome. Carfilzomib had antiproliferative and proapoptotic activities in vitro in solid and hematologic tumor cells. In animals, carfilzomib inhibited proteasome activity in blood and tissue and delayed tumor growth in models of multiple myeloma, hematologic, and solid tumors.
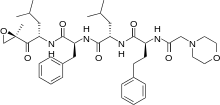
Structure
- Carfilzomib (carfilzomib) for Injection is an antineoplastic agent available for intravenous use only. Carfilzomib is a sterile, white to off-white lyophilized powder and is available as a single-use vial. Each vial of Carfilzomib contains 60 mg of carfilzomib, 3000 mg sulfobutylether beta-cyclodextrin, 57.7 mg citric acid, and sodium hydroxide for pH adjustment (target pH 3.5).
- Carfilzomib is a modified tetrapeptidyl epoxide, isolated as the crystalline free base. The chemical name for carfilzomib is (2S)-N-((S)-1-((S)-4-methyl-1-((R)-2-methyloxiran-2-yl)-1-oxopentan-2-ylcarbamoyl)-2-phenylethyl)-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)-4-methylpentanamide. Carfilzomib has the following structure:
- Carfilzomib is a crystalline substance with a molecular weight of 719.9. The molecular formula is C40H57N5O7. Carfilzomib is practically insoluble in water, and very slightly soluble in acidic conditions.
Pharmacodynamics
- Intravenous carfilzomib administration resulted in suppression of proteasome chymotrypsin-like activity when measured in blood 1 hour after the first dose. On Day 1 of Cycle 1, proteasome inhibition in peripheral blood mononuclear cells (PBMCs) ranged from 79% to 89% at 15 mg/m2, and from 82% to 83% at 20 mg/m2. In addition, carfilzomib administration resulted in inhibition of the LMP2 and MECL1 subunits of the immunoproteasome ranging from 26% to 32% and 41% to 49%, respectively, at 20 mg/m2. Proteasome inhibition was maintained for ≥ 48 hours following the first dose of carfilzomib for each week of dosing.
Pharmacokinetics
- Absorption: The Cmax and AUC following a single intravenous dose of 27 mg/m2 was 4232 ng/mL and 379 ng•hr/mL, respectively. Following repeated doses of carfilzomib at 15 and 20 mg/m2, systemic exposure (AUC) and half-life were similar on Days 1 and 15 or 16 of Cycle 1, suggesting there was no systemic carfilzomib accumulation. At doses between 20 and 36 mg/m2, there was a dose-dependent increase in exposure.
- Distribution: The mean steady-state volume of distribution of a 20 mg/m2 dose of carfilzomib was 28 L. When tested in vitro, the binding of carfilzomib to human plasma proteins averaged 97% over the concentration range of 0.4 to 4 micromolar.
- Metabolism: Carfilzomib was rapidly and extensively metabolized. The predominant metabolites measured in human plasma and urine, and generated in vitro by human hepatocytes, were peptide fragments and the diol of carfilzomib, suggesting that peptidase cleavage and epoxide hydrolysis were the principal pathways of metabolism. Cytochrome P450-mediated mechanisms played a minor role in overall carfilzomib metabolism. The metabolites have no known biologic activity.
- Elimination: Following intravenous administration of doses ≥ 15 mg/m2, carfilzomib was rapidly cleared from the systemic circulation with a half-life of ≤ 1 hour on Day 1 of Cycle 1. The systemic clearance ranged from 151 to 263 L/hour, and exceeded hepatic blood flow, suggesting that carfilzomib was largely cleared extrahepatically. The pathways of carfilzomib elimination have not been characterized in humans.
- Age: Analysis of population pharmacokinetics data after the first dose of Cycle 1 (Day 1) in 154 patients who had received an IV dose of 20 mg/m2 showed no clinically significant difference in exposure between patients < 65 years and ≥ 65 years of age.
- Gender: Mean dose-normalized AUC and Cmax values were comparable between male and female patients in the population pharmacokinetics study.
- Hepatic Impairment: No pharmacokinetic studies were performed with Carfilzomib in patients with hepatic impairment.
- Renal Impairment: A pharmacokinetic study was conducted in which 43 multiple myeloma patients who had various degrees of renal impairment and who were classified according to their creatinine clearances (CLcr) into the following groups: normal function (CLcr > 80 mL/min, n = 8), mild impairment (CLcr 50–80 mL/min, n = 12), moderate impairment (CLcr 30–49 mL/min, n = 8), severe impairment (CLcr < 30 mL/min, n = 7), and chronic dialysis (n = 8). Carfilzomib was administered intravenously over 2 to 10 minutes, on two consecutive days, weekly for three weeks (Days 1, 2, 8, 9, 15, and 16), followed by a 12-day rest period every 28 days. Patients received an initial dose of 15 mg/m2, which could be escalated to 20 mg/m2 starting in Cycle 2 if 15 mg/m2 was well tolerated in Cycle 1. In this study, renal function status had no effect on the clearance or exposure of carfilzomib following a single or repeat-dose administration.
- Cytochrome P450: In an in vitro study using human liver microsomes, carfilzomib showed modest direct and time-dependent inhibitory effect on human cytochrome CYP3A4/5. In vitro studies indicated that carfilzomib did not induce human CYP1A2 and CYP3A4 in cultured fresh human hepatocytes. Cytochrome P450-mediated mechanisms play a minor role in the overall metabolism of carfilzomib. A clinical trial of 17 patients using oral midazolam as a CYP3A probe demonstrated that the pharmacokinetics of midazolam were unaffected by concomitant carfilzomib administration. Carfilzomib is not expected to inhibit CYP3A4/5 activities and/or affect the exposure to CYP3A4/5 substrates.
- P-gp: Carfilzomib is a P-glycoprotein (P-gp) substrate and showed marginal inhibitory effects on P-gp in a Caco-2 monolayer system. Given that Carfilzomib is administrated intravenously and is extensively metabolized, the pharmacokinetic profile of Carfilzomib is unlikely to be affected by P-gp inhibitors or inducers.
Nonclinical Toxicology
- Carcinogenicity studies have not been conducted with carfilzomib.
- Carfilzomib was clastogenic in the in vitro chromosomal aberration test in peripheral blood lymphocytes. Carfilzomib was not mutagenic in the in vitro bacterial reverse mutation (Ames) test and was not clastogenic in the in vivo mouse bone marrow micronucleus assay.
- Fertility studies with carfilzomib have not been conducted. No effects on reproductive tissues were noted during 28-day repeat-dose rat and monkey toxicity studies or in 6-month rat and 9-month monkey chronic toxicity studies.
Clinical Studies
Relapsed Multiple Myeloma
- The safety and efficacy of Carfilzomib were evaluated in a single-arm, multicenter clinical trial. Two hundred and sixty-six patients with relapsed multiple myeloma who had received at least two prior therapies (including bortezomib and thalidomide and/or lenalidomide) were enrolled. Patients were enrolled in the trial whose disease had a less than or equal to 25% response to the most recent therapy or had disease progression during or within 60 days of the most recent therapy. Patients were excluded from the trial with total bilirubin levels ≥ 2 × upper limit of normal (ULN); creatinine clearance rates < 30 mL/min; New York Heart Association Class III to IV congestive heart failure; symptomatic cardiac ischemia; myocardial infarction within the last 6 months; peripheral neuropathy Grade 3 or 4, or peripheral neuropathy Grade 2 with pain; active infections requiring treatment; and pleural effusion.
- Carfilzomib was administered intravenously over 2 to 10 minutes on two consecutive days each week for three weeks, followed by a 12-day rest period (28-day treatment cycle), until disease progression, unacceptable toxicity, or for a maximum of 12 cycles. Patients received 20 mg/m2 at each dose in Cycle 1, and 27 mg/m2 in subsequent cycles. To reduce the incidence and severity of fever, rigors, chills, dyspnea, myalgia, and arthralgia, dexamethasone 4 mg by mouth or by intravenous infusion was administered prior to all Carfilzomib doses during the first cycle and prior to all Carfilzomib doses during the first dose-escalation (27 mg/m2) cycle. Dexamethasone premedication (4 mg orally or intravenously) was reinstated if these symptoms reappeared during subsequent cycles.
- Baseline patient and disease characteristics are summarized in Table 5.
- The median number of cycles started was four.
- The primary endpoint was the overall response rate (ORR) as determined by Independent Review Committee assessment using International Myeloma Working Group criteria. The ORR (stringent complete response [sCR] + complete response [CR] + very good partial response [VGPR] + partial response [PR]) was 22.9% (95% CI: 18.0, 28.5) (N = 266) (see Table 6). The median duration of response (DOR) was 7.8 months (95% CI: 5.6, 9.2).
How Supplied
- Carfilzomib (carfilzomib) for Injection is supplied as an individually cartoned single-use vial containing a dose of 60 mg of carfilzomib as a white to off-white lyophilized cake or powder.
- NDC 76075-101-01, 60 mg carfilzomib per vial
- Storage and Handling
- Unopened vials should be stored refrigerated (2°C to 8°C; 36°F to 46°F). Retain in original package to protect from light.
Storage
There is limited information regarding Carfilzomib Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Carfilzomib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Carfilzomib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Discuss the following with patients prior to treatment with Carfilzomib:
- Instruct patients to contact their physician if they develop any of the following symptoms: fever, chills, rigors, chest pain, cough, or swelling of the feet or legs.
- Advise patients that Carfilzomib may cause fatigue, dizziness, fainting, and/or drop in blood pressure. Advise patients not to drive or operate machinery if they experience any of these symptoms.
- Advise patients that they may experience shortness of breath (dyspnea) during treatment with Carfilzomib. This most commonly occurs within a day of dosing. Advise patients to contact their physicians if they experience shortness of breath.
- Counsel patients to avoid dehydration, since patients receiving Carfilzomib therapy may experience vomiting and/or diarrhea. Instruct patients to seek medical advice if they experience symptoms of dizziness, lightheadedness, or fainting spells.
- Counsel females of reproductive potential to use effective contraceptive measures to prevent pregnancy during treatment with Carfilzomib. Advise the patient that if she becomes pregnant during treatment, to contact her physician immediately. Advise patients not to take Carfilzomib treatment while pregnant or breastfeeding. If a patient wishes to restart breastfeeding after treatment, advise her to discus
Precautions with Alcohol
- Alcohol-Carfilzomib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- KYPROLIS®[1]
Look-Alike Drug Names
There is limited information regarding Carfilzomib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Carfilzomib
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Carfilzomib |Label Name=Carfilzomib07.png
}}
{{#subobject:
|Label Page=Carfilzomib |Label Name=Carfilzomib08.png
}}