Butorphanol (inhalation)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Butorphanol (inhalation) is an opioid analgesic that is FDA approved for the treatment of pain. Common adverse reactions include nausea, vomiting, dizziness, insomnia, sedation, somnolence, and nasal congestion.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Butorphanol Tartrate Nasal Spray USP is indicated for the management of pain when the use of an opioid analgesic is appropriate.
Dosage
Use for Pain
- The usual recommended dose for initial nasal administration is 1 mg (1 spray in one nostril). Adherence to this dose reduces the incidence of drowsiness and dizziness. If adequate pain relief is not achieved within 60 to 90 minutes, an additional 1 mg dose may be given.
- The initial dose sequence outlined above may be repeated in 3 to 4 hours as required after the second dose of the sequence.
- Depending on the severity of the pain, an initial dose of 2 mg (1 spray in each nostril) may be used in patients who will be able to remain recumbent in the event drowsiness or dizziness occurs. In such patients single additional 2 mg doses should not be given for 3 to 4 hours.
Use in Balanced Anesthesia
- The use of butorphanol tartrate nasal spray is not recommended because it has not been studied in induction or maintenance of anesthesia.
Labor
- The use of butorphanol tartrate nasal spray is not recommended as it has not been studied in labor.
Safety and Handling
- Butorphanol tartrate nasal spray is an open delivery system with increased risk of exposure to health care workers.
- In the priming process, a certain amount of butorphanol may be aerosolized; therefore, the pump sprayer should be aimed away from the patient or other people or animals.
- The disposal of Schedule IV controlled substances must be consistent with State and Federal Regulations. The unit should be disposed of by unscrewing the cap, rinsing the bottle, and placing the parts in a waste container.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Butorphanol (inhalation) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Butorphanol (inhalation) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Butorphanol (inhalation) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Butorphanol (inhalation) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Butorphanol (inhalation) in pediatric patients.
Contraindications
- Butorphanol Tartrate Nasal Spray USP is contraindicated in patients hypersensitive to butorphanol tartrate or the preservative benzethonium chloride.
Warnings
Patients Dependent on Narcotics
- Because of its opioid antagonist properties, butorphanol is not recommended for use in patients dependent on narcotics. Such patients should have an adequate period of withdrawal from opioid drugs prior to beginning butorphanol therapy. In patients taking opioid analgesics chronically, butorphanol has precipitated withdrawal symptoms such as anxiety, agitation, mood changes, hallucinations, dysphoria, weakness and diarrhea.
- Because of the difficulty in assessing opioid tolerance in patients who have recently received repeated doses of narcotic analgesic medication, caution should be used in the administration of butorphanol to such patients.
Drug Abuse and Dependence
Drug Abuse
- Butorphanol tartrate, by all routes of administration, has been associated with episodes of abuse. Of the cases received, there were more reports of abuse with the nasal spray formulation than with the injectable formulation.
Physical Dependence, Tolerance, and Withdrawal
- Prolonged, continuous use of butorphanol tartrate may result in physical dependence or tolerance (a decrease in response to a given dose). Abrupt cessation of use by patients with physical dependence may result in symptoms of withdrawal.
- Note: Proper patient selection, dose and prescribing limitations, appropriate directions for use, and frequent monitoring are important to minimize the risk of abuse and physical dependence.
Adverse Reactions
Clinical Trials Experience
Clinical Trial Experience
- A total of 2446 patients were studied in premarketing clinical trials of butorphanol. Approximately half received butorphanol tartrate injection with the remainder receiving butorphanol tartrate nasal spray. In nearly all cases the type and incidence of side effects with butorphanol by any route were those commonly observed with opioid analgesics.
- The adverse experiences described below are based on data from short-term and long-term clinical trials in patients receiving butorphanol by any route. There has been no attempt to correct for placebo effect or to subtract the frequencies reported by placebo-treated patients in controlled trials.
- The most frequently reported adverse experiences across all clinical trials with butorphanol tartrate injection and butorphanol tartrate nasal spray were somnolence (43%), dizziness (19%), nausea and/or vomiting (13%). In long-term trials with butorphanol tartrate nasal spray only, nasal congestion (13%) and insomnia (11%) were frequently reported.
- The following adverse experiences were reported at a frequency of 1% or greater in clinical trials, and were considered to be probably related to the use of butorphanol.
- Body as a Whole: asthenia/lethargy, headache, sensation of heat
- Cardiovascular: vasodilation, palpitations
- Digestive: anorexia, constipation, dry mouth, nausea and/or vomiting, stomach pain
- Nervous: anxiety, confusion, dizziness, euphoria, floating feeling, insomnia, nervousness, paresthesia, somnolence, tremor
- Respiratory: bronchitis, cough, dyspnea, epistaxis, nasal congestion, nasal irritation, pharyngitis, rhinitis, sinus congestion, sinusitis, upper respiratory infection
- Skin and Appendages: sweating/clammy, pruritus
- Special Senses: blurred vision, ear pain, tinnitus, unpleasant taste
- The following adverse experiences were reported with a frequency of less than 1% in clinical trials and were considered to be probably related to the use of butorphanol.
- Cardiovascular: hypotension, syncope
- Nervous: abnormal dreams, agitation, dysphoria, hallucinations, hostility, withdrawal symptoms
- Skin and Appendages: rash/hives
- Urogenital: impaired urination
- The following infrequent additional adverse experiences were reported in a frequency of less than 1% of the patients studied in short-term butorphanol tartrate nasal spray trials and under circumstances where the association between these events and butorphanol administration is unknown. They are being listed as alerting information for the physician.
- Body as a Whole: edema
- Cardiovascular: chest pain, hypertension, tachycardia
- Nervous: depression
- Respiratory: shallow breathing
Postmarketing Experience
- Postmarketing experience with butorphanol tartrate nasal spray and butorphanol tartrate injection has shown an adverse event profile similar to that seen during the premarketing evaluation of butorphanol by all routes of administration. Adverse experiences that were associated with the use of butorphanol tartrate nasal spray or butorphanol tartrate injection and that are not listed above have been chosen for inclusion below because of their seriousness, frequency of reporting, or probable relationship to butorphanol. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These adverse experiences include apnea, convulsion, delusion, drug dependence, excessive drug effect associated with transient difficulty speaking and/or executing purposeful movements, overdose, and vertigo. Reports of butorphanol overdose with a fatal outcome have usually but not always been associated with ingestion of multiple drugs.
Drug Interactions
- Concurrent use of butorphanol with central nervous system depressants (e.g., alcohol, barbiturates, tranquilizers, and antihistamines) may result in increased central nervous system depressant effects. When used concurrently with such drugs, the dose of butorphanol should be the smallest effective dose and the frequency of dosing reduced as much as possible when administered concomitantly with drugs that potentiate the action of opioids.
- In healthy volunteers, the pharmacokinetics of a 1 mg dose of butorphanol administered as butorphanol tartrate nasal spray were not affected by the coadministration of a single 6 mg subcutaneous dose of sumatriptan. However, in another study in healthy volunteers, the pharmacokinetics of butorphanol were significantly altered (29% decrease in AUC and 38% decreases in Cmax) when a 1 mg dose of butorphanol tartrate nasal spray was administered 1 minute after a 20 mg dose of sumatriptan nasal spray. (The two drugs were administered in opposite nostrils.) When the butorphanol tartrate nasal spray was administered 30 minutes after the sumatriptan nasal spray, the AUC of butorphanol increased 11% and Cmax decreased 18%.
- In neither case were the pharmacokinetics of sumatriptan affected by coadministration with butorphanol tartrate nasal spray. These results suggest that the analgesic effect of butorphanol tartrate nasal spray may be diminished when it is administered shortly after sumatriptan nasal spray, but by 30 minutes any such reduction in effect should be minimal.
- The safety of using Butorphanol Tartrate Nasal Spray and IMITREX®1 (sumatriptan) Nasal Spray during the same episode of migrane has not been established. However, it should be noted that both products are capable of producing transient increases in blood pressure.
- The pharmacokinetics of a 1 mg dose of butorphanol administered as butorphanol tartrate nasal spray were not affected by the coadministration of cimetidine (300 mg QID). Conversely, the administration of butorphanol tartrate nasal spray (1 mg butorphanol QID) did not alter the phamacokinetics of a 300 mg dose of cimetidine.
- It is not known if the effects of butorphanol are altered by concomitant medications that affect hepatic metabolism of drugs (erythromycin, theophylline, etc.), but physicians should be alert to the possibility that a smaller initial dose and longer intervals between doses may be needed.
- The fraction of butorphanol tartrate nasal spray absorbed is unaffected by the concomitant administration of a nasal vasoconstrictor (oxymetazoline), but the rate of absorption is decreased. Therefore, a slower onset can be anticipated if butorphanol tartrate nasal spray is administered concomitantly with, or immediately following, a nasal vasoconstrictor.
- No information is available about the use of butorphanol concurrently with MAO inhibitors
Use in Specific Populations
Pregnancy
- Reproduction studies in mice, rats and rabbits during organogenesis did not reveal any teratogenic potential to butorphanol. However, pregnant rats treated subcutaneously with butorphanol at 1 mg/kg (5.9 mg/m2) had a higher frequency of stillbirths than controls. Butorphanol at 30 mg/kg/oral (360 mg/m2) and 60 mg/kg/oral (720 mg/m2) also showed higher incidences of post-implantation loss in rabbits.
- There are no adequate and well-controlled studies of butorphanol tartrate in pregnant women before 37 weeks of gestation. Butorphanol tartrate should be used during pregnancy only if the potential benefit justifies the potential risk to the infant.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Butorphanol (inhalation) in women who are pregnant.
Labor and Delivery
Butorphanol tartrate nasal spray is not recommended during labor or delivery because there is no clinical experience with its use in this setting.
Nursing Mothers
- Butorphanol has been detected in milk following administration of butorphanol tartrate injection to nursing mothers. The amount an infant would receive is probably clinically insignificant (estimated 4 mcg/L of milk in a mother receiving 2 mg IM four times a day).
Although there is no clinical experience with the use of butorphanol tartrate nasal spray in nursing mothers, it should be assumed that butorphanol will appear in the milk in similar amounts following the nasal route of administration.
Pediatric Use
Butorphanol is not recommended for use in patients below 18 years of age because safety and efficacy have not been established in this population.
Geriatic Use
- Of the approximately 1700 patients treated with butorphanol tartrate nasal spray in clinical studies, 8% were 65 years of age or older and 2% were 75 years or older.
- Due to changes in clearance, the mean half-life of butorphanol is increased by 25% (to over 6 hours) in patients over the age of 65 years. Elderly patients may be more sensitive to the side effects of butorphanol. In clinical studies of butorphanol tartrate nasal spray, elderly patients had an increased frequency of headache, dizziness, drowsiness, vertigo, constipation, nausea and/or vomiting, and nasal congestion compared with younger patients. There are insufficient efficacy data for patients >65 years to determine whether they respond differently from younger patients.
- Initially a 1 mg dose of butorphanol tartrate nasal spray should generally be used in geriatric patients and 90 to 120 minutes should elapse before administering a second 1 mg dose, if needed.
- Butorphanol and its metabolites are known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection.
Gender
There is no FDA guidance on the use of Butorphanol (inhalation) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Butorphanol (inhalation) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Butorphanol (inhalation) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Butorphanol (inhalation) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Butorphanol (inhalation) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Butorphanol (inhalation) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Butorphanol (inhalation) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Butorphanol (inhalation) in the drug label.
Overdosage
Clinical Manifestations
- The clinical manifestations of butorphanol overdose are those of opioid drugs in general. Consequences of overdose vary with the amount of butorphanol ingested and individual response to the effects of opiates. The most serious symptoms are hypoventilation, cardiovascular insufficiency, coma, and death. Butorphanol overdose may be associated with ingestion of multiple drugs.
- Overdose can occur due to accidental or intentional misuse of butorphanol, especially in young children who may gain access to the drug in the home.
Treatment
- The management of suspected butorphanol overdosage includes maintenance of adequate ventilation, peripheral perfusion, normal body temperature, and protection of the airway. Patients should be under continuous observation with adequate serial measures of mental state, responsiveness and vital signs. Oxygen and ventilatory assistance should be available with continual monitoring by pulse oximetry if indicated. In the presence of coma, placement of an artificial airway may be required. An adequate intravenous portal should be maintained to facilitate treatment of hypotension associated with vasodilation.
- The use of a specific opioid antagonist such as naloxone should be considered. As the duration of butorphanol action usually exceeds the duration of action of naloxone, repeated dosing with naloxone may be required.
- In managing cases of suspected butorphanol overdosage, the possibility of multiple drug ingestion should always be considered.
Pharmacology
Mechanism of Action
- *Butorphanol is a mixed agonist-antagonist with low intrinsic activity at receptors of the µ-opioid type (morphine-like). It is also an agonist at K -opioid receptors. Its interactions with these receptors in the central nervous system apparently mediate most of its pharmacologic effects, including analgesia.
- In addition to analgesia, CNS effects include depression of spontaneous respiratory activity and cough, stimulation of the emetic center, miosis and sedation. Effects possibly mediated by non-CNS mechanisms include alteration in cardiovascular resistance and capacitance, bronchomotor tone, gastrointestinal secretory and motor activity and bladder sphincter activity.
- In an animal model, the dose of butorphanol tartrate required to antagonize morphine analgesia by 50% was similar to that for nalorphine, less than that for pentazocine and more than that for naloxone. The pharmacological activity of butorphanol metabolites has not been studied in humans; in animal studies, butorphanol metabolites have demonstrated some analgesic activity. In human studies of butorphanol, sedation is commonly noted at doses of 0.5 mg or more. Narcosis is produced by 10 to 12 mg doses of butorphanol administered over 10 to 15 minutes intravenously.
- Butorphanol, like other mixed agonist-antagonists with a high affinity for the ĸ-receptor, may produce unpleasant psychotomimetic effects in some individuals. Nausea and/or vomiting may be produced by doses of 1 mg or more administered by any route.
Structure
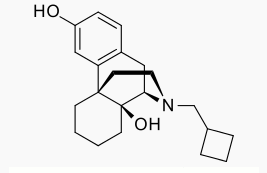
- Butorphanol tartrate is a synthetically derived opioid agonist-antagonist analgesic of the phenanthrene series. The chemical name is (-)-17-(cyclobutylmethyl)morphinan-3,14-diol[S-(R*,R*)]-2,3-dihydroxy-butanedioate (1:1) (salt). The molecular formula is C21H29NO2•C4H6O6, which corresponds to a molecular weight of 477.55 and the following structural formula:
Pharmacodynamics
- The analgesic effect of butorphanol is influenced by the route of administration. Onset of analgesia is within a few minutes for intravenous administration, within 15 minutes for intramuscular injection, and within 15 minutes for the nasal spray doses.
- Peak analgesic activity occurs within 30 to 60 minutes following intravenous and intramuscular administration and within 1 to 2 hours following the nasal spray administration.
- The duration of analgesia varies depending on the pain model as well as the route of administration, but is generally 3 to 4 hours with IM and IV doses as defined by the time 50% of patients required remedication. In postoperative studies, the duration of analgesia with IV or IM butorphanol was similar to morphine, meperidine, and pentazocine when administered in the same fashion at equipotent doses. Compared to the injectable form and other drugs in this class, butorphanol tartrate nasal spray has a longer duration of action (4 to 5 hours)
Pharmacokinetics

- Butorphanol is extensively metabolized in the liver. Metabolism is qualitatively and quantitatively similar following intravenous, intramuscular, or nasal administration. Oral bioavailability is only 5 to 17% because of extensive first pass metabolism of butorphanol.
- The major metabolite of butorphanol is hydroxybutorphanol, while norbutorphanol is produced in small amounts. Both have been detected in plasma following administration of butorphanol, with norbutorphanol present at trace levels at most time points. The elimination half-life of hydroxybutorphanol is about 18 hours and, as a consequence, considerable accumulation (~5-fold) occurs when butorphanol is dosed to steady state (1 mg transnasally q6h for 5 days).
- Elimination occurs by urine and fecal excretion. When 3H labelled butorphanol is administered to normal subjects, most (70 to 80%) of the dose is recovered in the urine, while approximately 15% is recovered in the feces.
- About 5% of the dose is recovered in the urine as butorphanol. Forty-nine percent is eliminated in the urine as hydroxybutorphanol. Less than 5% is excreted in the urine as norbutorphanol.
- Butorphanol pharmacokinetics in the elderly differ from younger patients (see Table 1). The mean absolute bioavailability of butorphanol tartrate nasal spray in elderly women (48%) was less than that in elderly men (75%), young men (68%), or young women (70%). Elimination half-life is increased in the elderly (6.6 hours as opposed to 4.7 hours in younger subjects).
- In renally impaired patients with creatinine clearances <30 mL/min, the elimination half-life was approximately doubled and the total body clearance was approximately one half (10.5 hours [clearance 150 L/h] as compared to 5.8 hours [clearance 260 L/h] in healthy subjects). No effect on Cmax or Tmax was observed after a single dose.
- After intravenous administration to patients with hepatic impairment, the elimination half-life of butorphanol was approximately tripled and total body clearance was approximately one half (half-life 16.8 hours, clearance 92 L/h) compared to healthy subjects (half-life 4.8 hours, clearance 175 L/h). The exposure of hepatically impaired patients to butorphanol was significantly greater (about 2-fold) than that in healthy subjects. Similar results were seen after nasal administration. No effect on Cmax or Tmax was observed after a single intranasal dose.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Butorphanol (inhalation) in the drug label.
Clinical Studies
- The effectiveness of opioid analgesics varies in different pain syndromes.
- Studies with butorphanol tartrate nasal spray have been performed in postoperative (general, orthopedic, oral, cesarean section) pain, in postepisiotomy pain, in pain of musculoskeletal origin, and in migraine headache pain (see below).
Use in the Management of Pain
- Postoperative Pain: The analgesic efficacy of butorphanol tartrate nasal spray was evaluated (approximately 35 patients per treatment group) in a general and orthopedic surgery trial. Single doses of butorphanol tartrate nasal spray (1 or 2 mg) and IM meperidine (37.5 or 75 mg) were compared. Analgesia provided by 1 and 2 mg doses of butorphanol tartrate nasal spray was similar to 37.5 and 75 mg meperidine, respectively, with onset of analgesia within 15 minutes and peak analgesic effect within 1 hour. The median duration of pain relief was 2.5 hours with 1 mg butorphanol tartrate nasal spray, 3.5 hours with 2 mg butorphanol tartrate nasal spray and 3.3 hours with either dose of meperidine.
- In a postcesarean section trial, butorphanol tartrate nasal spray administered to 35 patients as two 1 mg doses 60 minutes apart was compared with a single 2 mg dose of butorphanol tartrate nasal spray or a single 2 mg IV dose of butorphanol tartrate injection (37 patients each). Onset of analgesia was within 15 minutes for all butorphanol tartrate regimens. Peak analgesic effects of 2 mg intravenous butorphanol tartrate injection and butorphanol tartrate nasal spray were similar in magnitude. The duration of pain relief provided by both 2 mg butorphanol tartrate nasal spray regimens was approximately 4.5 hours and was greater than intravenous butorphanol tartrate injection (2.6 hours).
- Migraine Headache Pain: The analgesic efficacy of two 1 mg doses 1 hour apart of butorphanol tartrate nasal spray in migraine headache pain was compared with a single dose of 10 mg IM methadone (31 and 32 patients, respectively). Significant onset of analgesia occurred within 15 minutes for both butorphanol tartrate nasal spray and IM methadone. Peak analgesic effect occurred at 2 hours for butorphanol tartrate nasal spray and 1.5 hours for methadone. The median duration of pain relief was 6 hours with butorphanol tartrate nasal spray and 4 hours with methadone as judged by the time when approximately half of the patients remedicated.
- In two other trials in patients with migraine headache pain, a 2 mg initial dose of butorphanol tartrate nasal spray followed by an additional 1 mg dose 1 hour later (76 patients) was compared with either 75 mg IM meperidine (24 patients) or placebo (72 patients). Onset, peak activity and duration were similar with both active treatments; however, the incidence of adverse experiences (nausea, vomiting, dizziness) was higher in these two trials with the 2 mg initial dose of butorphanol tartrate nasal spray than in the trial with the 1 mg initial dose.
Individualization of Dosage
- Use of butorphanol in geriatric patients, patients with renal impairment, patient with hepatic impairment, and during labor requires extra caution.
- The usual recommended dose for initial nasal administration is 1 mg (1 spray in one nostril). If adequate pain relief is not achieved within 60 to 90 minutes, an additional 1 mg dose may be given.
- The initial dose sequence outlined above may be repeated in 3 to 4 hours as required after the second dose of the sequence.
- For the management of severe pain, an initial dose of 2 mg (1 spray in each nostril) may be used in patients who will be able to remain recumbent in the event drowsiness or dizziness occurs. In such patients additional doses should not be given for 3 to 4 hours. The incidence of adverse events is higher with an initial 2 mg dose.
- The initial dose sequence in elderly patients and patients with renal or hepatic impairment should be limited to 1 mg followed, if needed, by 1 mg in 90 to 120 minutes. The repeat dose sequence in these patients should be determined by the patient’s response rather than at fixed times but will generally be no less than at 6 hour intervals.
How Supplied
- Butorphanol Tartrate Nasal Spray USP is supplied in a child-resistant vial containing a 2.5 mL bottle of nasal spray solution (10 mg/mL) and a metered-dose spray pump with protective clip and dust cover, a bottle of nasal spray solution, and a patient instruction leaflet and medication guide. On average, one bottle will deliver 14 to 15 doses if no repriming is necessary.
- Butorphanol Tartrate Nasal Spray USP, 10 mg/mL
- NDC 0054-3090-36: 2.5 mL bottle.
Storage
- Store at 20° to 25°C (68° to 77°F). Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Images
Drug Images
{{#ask: Page Name::Butorphanol (inhalation) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Butorphanol (inhalation) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Butorphanol (inhalation) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- BUTORPHANOL TARTRATE®[1]
Look-Alike Drug Names
There is limited information regarding Butorphanol (inhalation) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "BUTORPHANOL TARTRATE- butorphanol tartrate spray, metered LA". line feed character in
|title=at position 59 (help)