Bartter syndrome pathophysiology
Main article: Bartter syndrome
|
Bartter syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Bartter syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Bartter syndrome pathophysiology |
|
Risk calculators and risk factors for Bartter syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Tayyaba Ali, M.D.[2]
Overview
The thick ascending limb of the loop of Henle is not permeable to water and reabsorbs a large proportion of the filtered sodium chloride as shown in the figure, which leads to interstitial hypertonicity that powers the countercurrent exchange and urinary concentration mechanisms. In case of impairment of this function, a major loss of water and sodium occurs, as seen with loop diuretics.Bartter syndrome is a renal tubular salt-wasting disorder in which the kidneys cannot reabsorb sodium and chloride in the thick ascending limb of the loop of Henle. Impairment of sodium and chloride reabsorption is the primary defect in the Bartter syndrome that initiates the cascade. This leads to increased delivery of salt to the distal tubules and excessive salt and water loss from the body. The resultant volume depletion causes activation of the renin-angiotensin-aldosterone system (RAAS) and subsequent secondary hyperaldosteronism. Long-term stimulation causes hyperplasia of the juxtaglomerular apparatus and elevates renin levels. Excessive distal delivery of sodium follows by sodium (Na) reabsorption in the distal convoluted tubule. Na reabsorption exchange with the secretion of positively charged potassium or hydrogen ion and leads to increased loss of potassium (K+) in urine and increased hydrogen (H+) secretion. Decreased chloride (Cl-) reabsorption decreases the exchange with bicarbonate (HCO3-). Thus, increased bicarbonate retention and hypokalemia result in metabolic alkalosis. Calcium and magnesium reabsorb in the ascending limb of the loop of Henle as a result of a positive electrochemical gradient in the lumen created by the back leak of K+ ion in the lumen, drives passive paracellular sodium, calcium, and magnesium reabsorption as shown in the figure. The defective sodium chloride transport in the ascending limb of the loop of Henle associated with Bartter syndrome leads to the impaired electrochemical gradient leading to increased urinary loss of calcium and magnesium. This leads to the development of nephrocalcinosis in Bartter syndrome.
Pathophysiology
- The thick ascending limb of the loop of Henle is not permeable to water and reabsorbs a large proportion of the filtered sodium chloride as shown in the figure, which leads to interstitial hypertonicity that powers the countercurrent exchange and urinary concentration mechanisms. In case of impairment of this function, a major loss of water and sodium occurs, as seen with loop diuretics.[1]
- Bartter syndrome is a renal tubular salt-wasting disorder in which the kidneys cannot reabsorb sodium and chloride in the thick ascending limb of the loop of Henle.
- Impairment of sodium and chloride reabsorption is the primary defect in the Bartter syndrome that initiates the cascade.
- This leads to increased delivery of salt to the distal tubules and excessive salt and water loss from the body. The resultant volume depletion causes activation of the renin-angiotensin-aldosterone system (RAAS) and subsequent secondary hyperaldosteronism. Long-term stimulation causes hyperplasia of the juxtaglomerular apparatus and elevates renin levels.[2][3]
- Excessive distal delivery of sodium follows by sodium (Na) reabsorption in the distal convoluted tubule. Na reabsorption exchange with the secretion of positively charged potassium or hydrogen ion and leads to increased loss of potassium (K+) in urine and increased hydrogen (H+) secretion.
- Decreased chloride (Cl-) reabsorption decreases the exchange with bicarbonate (HCO3-). Thus, increased bicarbonate retention and hypokalemia result in metabolic alkalosis.[4]
- Defective sodium reabsorption in the loop of Henle impairs the concentration of urine as water follows sodium.[5]
- Calcium and magnesium reabsorb in the ascending limb of the loop of Henle as a result of a positive electrochemical gradient in the lumen created by the back leak of K+ ion in the lumen, drives passive paracellular sodium, calcium, and magnesium reabsorption as shown in the figure. The defective sodium chloride transport in the ascending limb of the loop of Henle associated with Bartter syndrome leads to the impaired electrochemical gradient leading to increased urinary loss of calcium and magnesium. This leads to the development of nephrocalcinosis in Bartter syndrome.[6]
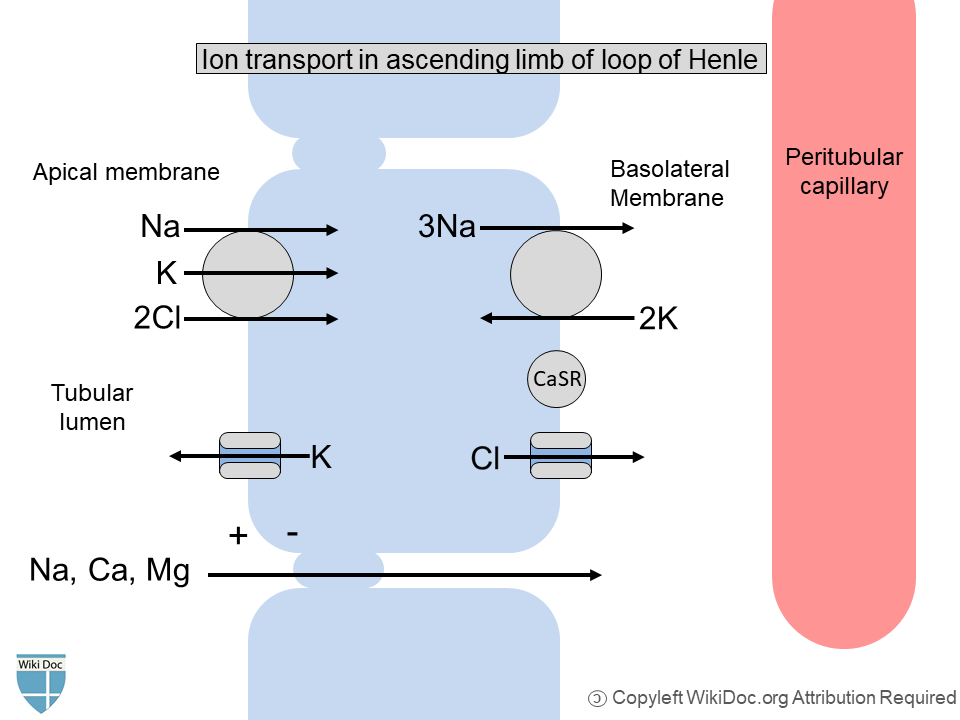
- Cl-channel kidney B (ClC-Kb) is the primary channel in the basolateral membrane of thick ascending limb for chloride reabsorption. Another chloride channel, Cl-channel kidney A (ClC-Ka), may have excess usefulness.
- Barttin is a small protein beta-subunit that collaborates with these chloride channels and enhances their functionality.
- These chloride channels also play an important role in ion transport in the ear, accounting for the association between some genetic renal transport defects and deafness. Neurosensory deafness can be a rare complication of Bartter syndrome when it is caused by specific mutations.[7]
- To browse the types of mutations responsible for different types of Bartter syndrome, click here.
- Hypokalemia has three further direct sequelae—increased production of PGE2 by renal cells, increased production of PGI2 by blood vessels, and decreased production of aldosterone by the adrenals. The increase in PGE2 stimulates renin production, which in turn results in increased angiotensin II and stimulation of aldosterone production by the adrenal gland.[8]
References
- ↑ Seyberth HW, Schlingmann KP (2011). "Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects". Pediatr Nephrol. 26 (10): 1789–802. doi:10.1007/s00467-011-1871-4. PMC 3163795. PMID 21503667.
- ↑ Deschênes G, Fila M (2011). "Primary molecular disorders and secondary biological adaptations in bartter syndrome". Int J Nephrol. 2011: 396209. doi:10.4061/2011/396209. PMC 3177086. PMID 21941653.
- ↑ BARTTER FC, PRONOVE P, GILL JR, MACCARDLE RC (1962). "Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome". Am J Med. 33: 811–28. doi:10.1016/0002-9343(62)90214-0. PMID 13969763.
- ↑ Soylu Ustkoyuncu P, Nalcacioglu H, Bastug F, Yel S, Altuner Torun Y (2019). "Association of Mucopolysaccharidosis Type 4A and Bartter Syndrome". Iran J Kidney Dis. 13 (1): 71–72. PMID 30851722.
- ↑ Al Shibli A, Narchi H (2015). "Bartter and Gitelman syndromes: Spectrum of clinical manifestations caused by different mutations". World J Methodol. 5 (2): 55–61. doi:10.5662/wjm.v5.i2.55. PMC 4482822. PMID 26140272.
- ↑ Seyberth HW (2008). "An improved terminology and classification of Bartter-like syndromes". Nat Clin Pract Nephrol. 4 (10): 560–7. doi:10.1038/ncpneph0912. PMID 18695706.
- ↑ Andrini O, Keck M, Briones R, Lourdel S, Vargas-Poussou R, Teulon J (2015). "ClC-K chloride channels: emerging pathophysiology of Bartter syndrome type 3". Am J Physiol Renal Physiol. 308 (12): F1324–34. doi:10.1152/ajprenal.00004.2015. PMID 25810436.
- ↑ "www.kidney-international.org".