Azithromycin microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Microbiology
Azithromycin acts by binding to the 50S ribosomal subunit of susceptible microorganisms and, thus, interfering with microbial protein synthesis. Nucleic acid synthesis is not affected.
Azithromycin concentrates in phagocytes and fibroblasts as demonstrated by in vitro incubation techniques. Using such methodology, the ratio of intracellular to extra-cellular concentration was >30 after one hour incubation. In vivo studies suggest that concentration in phagocytes may contribute to drug distribution to inflamed tissues.
Azithromycin has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section of the package insert for ZITHROMAX (azithromycin for injection).
Aerobic and Facultative Gram-Positive Microorganisms
NOTE: Azithromycin demonstrates cross-resistance with erythromycin-resistant gram-positive strains. Most strains of Enterococcus faecalis and methicillin-resistant staphylococci are resistant to azithromycin.
Aerobic and Facultative Gram-Negative Microorganisms
Other Microorganisms
- Chlamydia pneumoniae
- Chlamydia trachomatis
- Legionella pneumophila
- Mycoplasma hominis
- Mycoplasma pneumoniae
Beta-lactamase production should have no effect on azithromycin activity.
Azithromycin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section of the package insert for ZITHROMAX (azithromycin tablets) and ZITHROMAX (azithromycin for oral suspension).
Aerobic and Facultative Gram-Positive Microorganisms
Aerobic and Facultative Gram-Negative Microorganisms
Other Microorganisms
Beta-lactamase production should have no effect on azithromycin activity.
The following in vitro data are available, but their clinical significance is unknown.
At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoints for azithromycin. However, the safety and effectiveness of azithromycin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Aerobic and Facultative Gram-Positive Microorganisms
- Streptococci (Groups C, F, G)
- Viridans group streptococci
Aerobic and Facultative Gram-Negative Microorganisms
Anaerobic Microorganisms
Other Microorganisms
Beta-lactamase production should have no effect on azithromycin activity.
Susceptibility Testing Methods
When available, the results of in vitro susceptibility test results for antimicrobial drugs used in resident hospitals should be provided to the physician as periodic reports which describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports may differ from susceptibility data obtained from outpatient use, but could aid the physician in selecting the most effective antimicrobial.
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1,3 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of azithromycin powder. The MIC values should be interpreted according to criteria provided in Table 3.
Diffusion Technique
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2,3 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 15-µg azithromycin to test the susceptibility of microorganisms to azithromycin. The disk diffusion interpretive criteria are provided in Table 3.
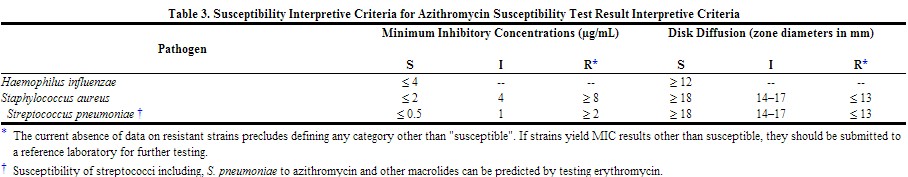 |
No interpretive criteria have been established for testing Neisseria gonorrhoeae. This species is not usually tested.
A report of "susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound reaches the concentrations usually achievable. A report of "intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound reaches the concentrations usually achievable; other therapy should be selected.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.