Atezolizumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Atezolizumab is a programmed death-ligand 1 (PD-L1) blocking antibody that is FDA approved for the treatment of elected patients (see below) with locally advanced or metastatic urothelial carcinoma. This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. Atezolizumab is also indicated for the treatment of patients with metastatic non-small cell lung cancer who have disease progression during or following platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving Atezolizumab.. Common adverse reactions include fatigue, decreased appetite, nausea, urinary tract infection, pyrexia, and constipation (≥20%) in patients with locally advanced or metastatic urothelial carcinoma. Most common adverse reactions (≥ 20%) in patients with metastatic non-small cell lung cancer were fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Locally Advanced or Metastatic Urothelial Carcinoma
Atezolizumab is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who:
- Have disease progression during or following platinum-containing chemotherapy
- Have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy
This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Atezolizumab is indicated for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) who have disease progression during or following platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving Atezolizumab.
Dosage
- Recommended Dosing
The recommended dose of Atezolizumab is 1200 mg administered as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity. If the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes. Do not administer Atezolizumab as an intravenous push or bolus.
- Dose Modifications
No dose reductions of Atezolizumab are recommended.
Withhold Atezolizumab for any of the following:
- Grade 2 pneumonitis
- Aspartate aminotransferase (AST) or alanine aminotransferase (ALT) greater than 3 and up to 5 times upper limit of normal (ULN) or total bilirubin greater than 1.5 and up to 3 times ULN
- Grade 2 or 3 diarrhea or colitis
- Symptomatic hypophysitis, adrenal insufficiency, hypothyroidism, hyperthyroidism, or Grade 3 or 4 hyperglycemia
- Grade 2 ocular inflammatory toxicity
- Grade 2 or 3 pancreatitis, or Grade 3 or 4 increases in amylase or lipase levels (greater than 2.0 times ULN)
- Grade 3 or 4 infection
- Grade 2 infusion-related reactions
- Grade 3 rash
Atezolizumab may be resumed in patients whose adverse reactions recover to Grade 0–1.
Permanently discontinue Atezolizumab for any of the following:
- Grade 3 or 4 pneumonitis
- AST or ALT greater than 5 times ULN or total bilirubin greater than 3 times ULN
- Grade 4 diarrhea or colitis
- Grade 4 hypophysitis
- Myasthenic syndrome/myasthenia gravis, Guillain-Barré or meningoencephalitis (all grades)
- Grade 3 or 4 ocular inflammatory toxicity
- Grade 4 or any grade of recurrent pancreatitis
- Grade 3 or 4 infusion-related reactions
- Grade 4 rash
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Atezolizumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Atezolizumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Atezolizumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Atezolizumab in pediatric patients.
Contraindications
None
Warnings
Immune-Related Pneumonitis
Immune-mediated pneumonitis or interstitial lung disease, defined as requiring use of corticosteroids and with no clear alternate etiology, occurred in patients receiving Atezolizumab . Monitor patients for signs with radiographic imaging and for symptoms of pneumonitis. Administer steroids at a dose of 1 to 2 mg/kg/day prednisone equivalents for Grade 2 or greater pneumonitis, followed by corticosteroid taper. Withhold Atezolizumab until resolution for Grade 2 pneumonitis. Permanently discontinue Atezolizumab for Grade 3 or 4 pneumonitis.
Across clinical trials, 2.6% (51/1978) of patients developed pneumonitis. Fatal pneumonitis occurred in two patients.
In 523 patients with urothelial carcinoma who received Atezolizumab, pneumonitis occurred in six (1.1%) patients. Of these patients, there was one patient with fatal pneumonitis, one patient with Grade 3, three patients with Grade 2, and one patient with Grade 1 pneumonitis. Atezolizumab was held in all cases and five patients were treated with corticosteroids. Pneumonitis resolved in three patients. The median time to onset was 2.6 months (range: 15 days to 4.2 months). The median duration was 15 days (range: 6 days to 3.1+ months).
In 1027 patients with NSCLC who received Atezolizumab, pneumonitis occurred in 38 (3.7%) patients. Of these patients, there was one patient with fatal pneumonitis, two patients with Grade 4, thirteen patients with Grade 3, eleven patients with Grade 2, and eleven patients with Grade 1 pneumonitis. Atezolizumab was held in 24 patients and 21 patients were treated with corticosteroids. Pneumonitis resolved in 26 of the 38 patients. The median time to onset was 3.3 months (range: 3 days to 18.7 months). The median duration was 1.4 months (range: 0 days to 12.6+ months).
Immune-Related Hepatitis
Immune-mediated hepatitis, defined as requiring use of corticosteroids and with no clear alternate etiology, occurred in patients receiving Atezolizumab treatment. Liver test abnormalities occurred in patients who received Atezolizumab . Monitor patients for signs and symptoms of hepatitis. Monitor AST, ALT, and bilirubin prior to and periodically during treatment with Atezolizumab. Administer corticosteroids at a dose of 1–2 mg/kg/day prednisone equivalents for Grade 2 or greater transaminase elevations, with or without concomitant elevation in total bilirubin, followed by corticosteroid taper. Withhold Atezolizumab for Grade 2 and permanently discontinue Atezolizumab for Grade 3 or 4 immune-mediated hepatitis.
Across clinical trials (n=1978), Grade 3 or 4 elevation occurred in ALT (2.5%), AST (2.3%), and total bilirubin (1.6%).
In patients with urothelial carcinoma (n=523), Grade 3 or 4 elevation occurred in ALT (2.5%), AST (2.5%), and total bilirubin (2.1%). Immune-mediated hepatitis occurred in 1.3% of patients. Of these cases, one patient died from hepatitis, five patients had Grade 3, and one patient had Grade 2 hepatitis. The median time to onset was 1.1 months (range: 0.4 to 7.7 months). Atezolizumab was temporarily interrupted in four patients; none of these patients developed recurrence of hepatitis after resuming Atezolizumab.
In patients with NSCLC, Grade 3 or 4 elevation occurred in ALT (1.4%), AST (1.3%), and total bilirubin (0.6%). Immune-mediated hepatitis occurred in 0.9% (9/1027) of patients. Of these nine patients, one patient had Grade 4, four patients had Grade 3, three patients had Grade 2, and one patient had Grade 1 immune-mediated hepatitis. The median time to onset was 28 days (range: 15 days to 4.2 months). Atezolizumab was temporarily interrupted in seven patients; none of these patients developed recurrence of hepatitis after resuming Atezolizumab.
Immune-Related Colitis
Immune-mediated colitis or diarrhea, defined as requiring use of corticosteroids and with no clear alternate etiology, occurred in patients receiving Atezolizumab. Monitor patients for signs and symptoms of diarrhea or colitis. Withhold treatment with Atezolizumab for Grade 2 diarrhea or colitis. If symptoms persist for longer than 5 days or recur, administer 1–2 mg/kg prednisone or equivalent per day. Withhold treatment with Atezolizumab for Grade 3 diarrhea or colitis. Treat with IV methylprednisolone 1–2 mg/kg per day and convert to oral steroids once the patient has improved. For both Grade 2 and Grade 3 diarrhea or colitis, when symptoms improve to Grade 0 or Grade 1, taper steroids over ≥ 1 month. Resume treatment with Atezolizumab if the event improves to Grade 0 or 1 within 12 weeks and corticosteroids have been reduced to the equivalent of ≤ 10 mg oral prednisone per day. Permanently discontinue Atezolizumab for Grade 4 diarrhea or colitis.
Across clinical trials, colitis or diarrhea occurred in 19.7% (389/1978) of all patients.
In 523 patients with urothelial carcinoma who received Atezolizumab, colitis or diarrhea occurred in 98 (18.7%) patients. Ten patients (1.9%) developed Grade 3 or 4 diarrhea. Four patients (0.8%) had immune-mediated colitis or diarrhea with a median time to onset of 1.7 months (range: 1.1 to 3.1 months). Immune-mediated colitis resolved with corticosteroid administration in three of these patients, while the other patient died without resolution of colitis in the setting of diarrhea-associated renal failure.
In 1027 patients with NSCLC who received Atezolizumab, colitis or diarrhea occurred in 198 (19.3%) patients. Twelve patients (1.2%) developed Grade 3 colitis or diarrhea. Five patients (0.5%) had immune-mediated colitis or diarrhea with a median time to onset of 21 days (range: 12 days to 3.4 months). Of these patients, one had Grade 3, two had Grade 2, and two had Grade 1 immune-mediated colitis or diarrhea. Immune-mediated colitis or diarrhea resolved with corticosteroid administration in four of these patients, while the fifth patient died due to disease progression prior to resolution of colitis.
Immune-Related Endocrinopathies
Immune-related thyroid disorders, adrenal insufficiency, and type 1 diabetes mellitus, including diabetic ketoacidosis, have occurred in patients receiving Atezolizumab. Monitor patients for clinical signs and symptoms of endocrinopathies.
Hypophysitis occurred in 0.2% (1/523) of patients with urothelial cancer receiving Atezolizumab. Monitor for signs and symptoms of hypophysitis. Administer corticosteroids and hormone replacement as clinically indicated. Withhold Atezolizumab for Grade 2 or Grade 3 and permanently discontinue for Grade 4 hypophysitis.
- Thyroid Disorders
Thyroid function was assessed routinely only at baseline and the end of the study. Monitor thyroid function prior to and periodically during treatment with Atezolizumab. Asymptomatic patients with abnormal thyroid function tests can receive Atezolizumab. For symptomatic hypothyroidism, withhold Atezolizumab and initiate thyroid hormone replacement as needed. Manage isolated hypothyroidism with replacement therapy and without corticosteroids. For symptomatic hyperthyroidism, withhold Atezolizumab and initiate an anti-thyroid drug as needed. Resume treatment with Atezolizumab when symptoms of hypothyroidism or hyperthyroidism are controlled and thyroid function is improving.
Across clinical trials, hypothyroidism and hyperthyroidism occurred in 3.9% (77/1978) and 1.0% (20/1978) of patients, respectively.
In 523 patients with urothelial carcinoma who received Atezolizumab, hypothyroidism occurred in 2.5% (13/523). One patient had Grade 3 and twelve patients had Grade 1–2 hypothyroidism. The median time to first onset was 5.4 months (range: 21 days to 11.3 months). Thyroid stimulating hormone (TSH) was elevated and above the patient's baseline in 16% (21/131) of patients with a follow-up measurement.
Hyperthyroidism occurred in 0.6% (3/523) of patients with urothelial carcinoma. Of the three urothelial carcinoma patients, one patient had Grade 2 and two patients had Grade 1 hyperthyroidism. The median time to onset was 3.2 months (range: 1.4 to 5.8 months). TSH was decreased and below the patient's baseline in 3.8% (5/131) of patients with a follow-up measurement.
In 1027 patients with NSCLC who received Atezolizumab, hypothyroidism occurred in 4.2% (43/1027). Three patients had Grade 3 and forty patients had Grade 1–2 hypothyroidism. The median time to onset was 4.8 months (range 15 days to 31 months.) TSH was elevated and above the patient's baseline in 17% (54/315) of patients with follow-up measurement.
Hyperthyroidism occurred in 1.1% (11/1027) of patients with NSCLC. Eight patients had Grade 2 and three patients had Grade 1 hyperthyroidism. The median time to onset was 4.9 months (range: 21 days to 31 months). TSH was decreased and below the patient's baseline in 7.6% (24/315) of patients with a follow-up measurement.
Adrenal insufficiency occurred in 0.4% (7/1978) of patients across clinical trials, including two patients with Grade 3, four patients with Grade 2, and one patient with Grade 1. Adrenal insufficiency resolved in two patients.
For symptomatic adrenal insufficiency, withhold Atezolizumab and administer methylprednisolone 1–2 mg/kg per day IV followed by oral prednisone 1–2 mg/kg per day or equivalent once symptoms improve. Start steroid taper when symptoms improve to ≤ Grade 1 and taper steroids over ≥ 1 month. Resume treatment with Atezolizumab if the event improves to ≤ Grade 1 within 12 weeks and corticosteroids have been reduced to the equivalent of ≤ 10 mg oral prednisone per day and the patient is stable on replacement therapy, if required.
New onset diabetes with ketoacidosis has occurred in patients receiving Atezolizumab. Diabetes mellitus without an alternative etiology occurred in one (0.2%) patient with urothelial carcinoma and three (0.3%) patients with NSCLC.
Initiate treatment with insulin for type 1 diabetes mellitus. For ≥ Grade 3 hyperglycemia (fasting glucose >250–500 mg/dL), withhold Atezolizumab. Resume treatment with Atezolizumab when metabolic control is achieved on insulin replacement therapy.
Other Immune-Related Adverse Reactions
Other immune-related adverse reactions including meningoencephalitis, myasthenic syndrome/myasthenia gravis, Guillain-Barré, ocular inflammatory toxicity, and pancreatitis, including increases in serum amylase and lipase levels, have occurred in ≤ 1.0% of patients treated with Atezolizumab.
Monitor patients for clinical signs and symptoms of meningitis or encephalitis. Permanently discontinue Atezolizumab for any grade of meningitis or encephalitis. Treat with IV steroids (1–2 mg/kg/day methylprednisolone or equivalent) and convert to oral steroids (prednisone 60 mg/day or equivalent) once the patient has improved. When symptoms improve to ≤ Grade 1, taper steroids over ≥ 1 month.
- Motor and Sensory Neuropathy
Monitor patients for symptoms of motor and sensory neuropathy. Permanently discontinue Atezolizumab for any grade of myasthenic syndrome/myasthenia gravis or Guillain-Barré syndrome. Institute medical intervention as appropriate. Consider initiation of systemic corticosteroids at a dose of 1–2 mg/kg/day prednisone.
Symptomatic pancreatitis without an alternative etiology occurred in 0.1% (2/1978) of patients across clinical trials. Monitor patients for signs and symptoms of acute pancreatitis. Withhold Atezolizumab for ≥ Grade 3 serum amylase or lipase levels (> 2.0 ULN), or Grade 2 or 3 pancreatitis. Treat with 1–2 mg/kg IV methylprednisolone or equivalent per day. Once symptoms improve, follow with 1–2 mg/kg of oral prednisone or equivalent per day. Resume treatment with Atezolizumab when serum amylase and lipase levels improve to ≤ Grade 1 within 12 weeks or symptoms of pancreatitis have resolved, and corticosteroids have been reduced to ≤ 10 mg oral prednisone or equivalent per day. Permanently discontinue Atezolizumab for Grade 4 or any grade of recurrent pancreatitis.
Infection
Severe infections, including sepsis, herpes encephalitis, and mycobacterial infection leading to retroperitoneal hemorrhage occurred in patients receiving Atezolizumab. Monitor patients for signs and symptoms of infection and treat with antibiotics for suspected or confirmed bacterial infections. Withhold Atezolizumab for ≥ Grade 3 infection.
Across clinical trials, infections occurred in 38.4% (759/1978) of patients.
In 523 patients with urothelial carcinoma who received Atezolizumab, infection occurred in 197 (37.7%) patients. Grade 3 or 4 infection occurred in sixty (11.5%) patients, while three patients died due to infections. Urinary tract infections were the most common cause of Grade 3 or higher infection, occurring in 37 (7.1%) patients.
In Study 3, a randomized trial in patients with NSCLC, infections were more common in patients treated with Atezolizumab(43%) compared with those treated with docetaxel (34%). Grade 3 or 4 infections occurred in 9.2% of patients treated with Atezolizumab compared with 2.2% in patients treated with docetaxel. Two patients (1.4%) treated with Atezolizumab and three patients (2.2%) treated with docetaxel died due to infection. Pneumonia was the most common cause of Grade 3 or higher infection, occurring in 7.7% of patients treated with Atezolizumab.
Infusion-Related Reactions
Severe infusion reactions have occurred in patients in clinical trials of Atezolizumab. Infusion-related reactions occurred in 1.3% (25/1978) of patients across clinical trials, 1.7% (9/523) of patients with urothelial carcinoma, and 1.6% (16/1027) of patients with NSCLC. Interrupt or slow the rate of infusion in patients with mild or moderate infusion reactions. Permanently discontinue Atezolizumab in patients with Grade 3 or 4 infusion reactions.
Embryo-Fetal Toxicity
Based on its mechanism of action, Atezolizumab can cause fetal harm when administered to a pregnant woman. Animal studies have demonstrated that inhibition of the PD-L1/PD-1 pathway can lead to increased risk of immune-related rejection of the developing fetus resulting in fetal death. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, advise the patient of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Atezolizumab and for at least 5 months after the last dose.
Adverse Reactions
Clinical Trials Experience
The following adverse reactions are discussed in greater detail in other sections of the label:
- Immune-Related Pneumonitis
- Immune-Related Hepatitis
- Immune-Related Colitis
- Immune-Related Endocrinopathies
- Other Immune-Related Adverse Reactions
- Infection
- Infusion-Related Reactions
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in Table 1 reflects exposure to Atezolizumab in Cohort 2 of Study 1. This cohort enrolled 310 patients in a single arm trial with locally advanced or metastatic urothelial carcinoma who had disease progression during or following at least one platinum-containing chemotherapy regimen or who had disease progression within 12 months of treatment with a platinum-containing neoadjuvant or adjuvant chemotherapy regimen. Patients received 1200 mg of Atezolizumab intravenously every 3 weeks until unacceptable toxicity or either radiographic or clinical progression. The median duration of exposure was 12.3 weeks (range: 0.1, 46 weeks).
The most common adverse reactions (≥ 20%) were fatigue (52%), decreased appetite (26%), nausea (25%), urinary tract infection (22%), pyrexia (21%), and constipation (21%). The most common Grade 3–4 adverse reactions (≥ 2%) were urinary tract infection, anemia, fatigue, dehydration, intestinal obstruction, urinary obstruction, hematuria, dyspnea, acute kidney injury, abdominal pain, venous thromboembolism, sepsis, and pneumonia.
Three patients (0.9%) who were treated with Atezolizumab experienced either sepsis, pneumonitis, or intestinal obstruction which led to death. Atezolizumab was discontinued for adverse reactions in 3.2% (10/310) of the 310 patients. Sepsis led to discontinuation in 0.6% (2/310) of patients. Adverse reactions leading to interruption of Atezolizumab occurred in 27% of patients; the most common (> 1%) were liver enzyme increase, urinary tract infection, diarrhea, fatigue, confusional state, urinary obstruction, pyrexia, dyspnea, venous thromboembolism, and pneumonitis. Serious adverse reactions occurred in 45% of patients. The most frequent serious adverse reactions (> 2%) were urinary tract infection, hematuria, acute kidney injury, intestinal obstruction, pyrexia, venous thromboembolism, urinary obstruction, pneumonia, dyspnea, abdominal pain, sepsis, and confusional state.
Table 1 summarizes the adverse reactions that occurred in ≥ 10% of patients while Table 2 summarizes Grade 3–4 selected laboratory abnormalities that occurred in ≥ 1% of patients treated with Atezolizumab in Cohort 2 of Study 1.
- Table 1: All Grade Adverse Reactions in ≥ 10% of Patients with Urothelial Carcinoma in Study 1

TECENTRIQ: Atezolizumab's Brand name
- Table 2: Grade 3–4 Laboratory Abnormalities in Patients with Urothelial Carcinoma in Study 1 in ≥ 1% of Patients
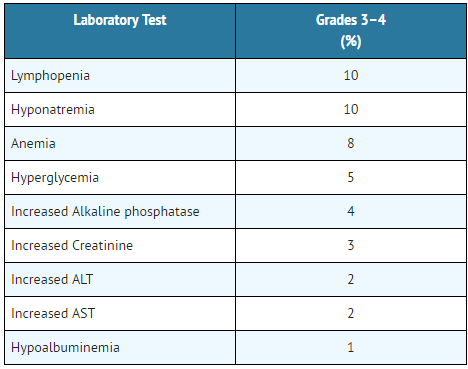
The safety of Atezolizumab was evaluated in Study 3, a multi-center, international, randomized, open-label trial in patients with metastatic NSCLC who progressed during or following a platinum-containing regimen, regardless of PD-L1 expression. Patients received 1200 mg of Atezolizumab(n=142) administered intravenously every 3 weeks until unacceptable toxicity or either radiographic or clinical progression or docetaxel (n=135) administered intravenously at 75 mg/m2 every 3 weeks until unacceptable toxicity or disease progression. The median duration of exposure was 3.7 months (range: 0–19 months) in Atezolizumab-treated patients and 2.1 months (range: 0–17 months) in docetaxel-treated patients.
The most common adverse reactions (≥ 20%) in patients receiving Atezolizumab were fatigue (46%), decreased appetite (35%), dyspnea (32%), cough (30%), nausea (22%), musculoskeletal pain (22%), and constipation (20%). The most common Grade 3-4 adverse reactions (≥2%) were dyspnea, pneumonia, hypoxia, hyponatremia, fatigue, anemia, musculoskeletal pain, AST increase, ALT increase, dysphagia, and arthralgia.
Nine patients (6.3%) who were treated with Atezolizumab experienced either pulmonary embolism (2), pneumonia (2), pneumothorax, ulcer hemorrhage, cachexia secondary to dysphagia, myocardial infarction, or large intestinal perforation which led to death. Atezolizumab was discontinued due to adverse reactions in 4% (6/142) of patients. Adverse reactions leading to interruption of Atezolizumab occurred in 24% of patients; the most common (>1%) were pneumonia, liver function test abnormality, upper respiratory tract infection, pneumonitis, acute kidney injury, hypoxia, hypothyroidism, dyspnea, anemia, and fatigue. Serious adverse reactions occurred in 37% of patients. The most frequent serious adverse reactions (> 2%) were pneumonia, dyspnea, pleural effusion, pyrexia, and venous thromboembolism.
Table 3 summarizes adverse reactions that occurred in at least 10% of Atezolizumab-treated patients and at a higher incidence than in the docetaxel arm. Table 4 summarizes selected laboratory abnormalities worsening from baseline that occurred in ≥10% of Atezolizumab-treated patients and at a higher incidence than in the docetaxel arm.

TECENTRIQ: Atezolizumab's Brand name

TECENTRIQ: Atezolizumab's Brand name
As with all therapeutic proteins, there is a potential for immunogenicity. Among 275 patients in Study 1, 114 patients (41.5%) tested positive for treatment-emergent (treatment-induced or treatment-enhanced) anti-therapeutic antibodies (ATA) at one or more post-dose time points. Among 135 patients in Study 3, 73 patients (54.1%) tested positive for treatment-emergent (treatment-induced or treatment-enhanced) anti-therapeutic antibodies (ATA) at one or more post-dose time points. In Study 1 and Study 3, the presence of ATAs did not appear to have a clinically significant impact on pharmacokinetics, safety or efficacy.
Immunogenicity assay results are highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications and underlying disease. For these reasons, comparison of incidence of ATAs to Atezolizumab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Atezolizumab Postmarketing Experience in the drug label.
Drug Interactions
The drug interaction potential of Atezolizumab is unknown.
Use in Specific Populations
Pregnancy
- Risk Summary
Based on its mechanism of action, Atezolizumab can cause fetal harm when administered to a pregnant woman. There are no available data on the use of Atezolizumab in pregnant women. Animal studies have demonstrated that inhibition of the PD-L1/PD-1 pathway can lead to increased risk of immune-related rejection of the developing fetus resulting in fetal death. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, advise the patient of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
- Data
- Animal Data
Animal reproduction studies have not been conducted with Atezolizumab to evaluate its effect on reproduction and fetal development. A literature-based assessment of the effects on reproduction demonstrated that a central function of the PD-L1/PD-1 pathway is to preserve pregnancy by maintaining maternal immune tolerance to a fetus. Blockage of PD-L1 signaling has been shown in murine models of pregnancy to disrupt tolerance to a fetus and to result in an increase in fetal loss; therefore, potential risks of administering Atezolizumab during pregnancy include increased rates of abortion or stillbirth. As reported in the literature, there were no malformations related to the blockade of PD-L1/PD-1 signaling in the offspring of these animals; however, immune-mediated disorders occurred in PD-1 and PD-L1 knockout mice. Based on its mechanism of action, fetal exposure to Atezolizumab may increase the risk of developing immune-mediated disorders or altering the normal immune response.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Atezolizumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Atezolizumab during labor and delivery.
Nursing Mothers
There is no information regarding the presence of Atezolizumab in human milk, the effects on the breastfed infant, or the effects on milk production. As human IgG is excreted in human milk, the potential for absorption and harm to the infant is unknown. Because of the potential for serious adverse reactions in breastfed infants from Atezolizumab, advise a lactating woman not to breastfeed during treatment and for at least 5 months after the last dose.
Pediatric Use
The safety and effectiveness of Atezolizumab have not been established in pediatric patients.
Geriatic Use
Of the 310 patients with urothelial carcinoma treated with Atezolizumab in Study 1, 59% were 65 years or older. Of the 142 patients with NSCLC treated with Atezolizumab in Study 3, 39% were 65 years or older. No overall differences in safety or efficacy were observed between patients ≥ 65 years of age and younger patients.
Gender
There is no FDA guidance on the use of Atezolizumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Atezolizumab with respect to specific racial populations.
Renal Impairment
Based on a population pharmacokinetic analysis, no dose adjustment of Atezolizumab is recommended for patients with renal impairment.
Hepatic Impairment
Based on a population pharmacokinetic analysis, no dose adjustment of Atezolizumab is recommended for patients with mild hepatic impairment. Atezolizumab has not been studied in patients with moderate or severe hepatic impairment
Females of Reproductive Potential and Males
- Females
Based on its mechanism of action, Atezolizumab can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with Atezolizumab and for at least 5 months following the last dose.
- Females
Based on animal studies, Atezolizumab may impair fertility in females of reproductive potential while receiving treatment
Immunocompromised Patients
There is no FDA guidance one the use of Atezolizumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Administer the initial infusion over 60 minutes through an intravenous line with or without a sterile, non-pyrogenic, low-protein binding in-line filter (pore size of 0.2–0.22 micron). If the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes.
Do not co-administer other drugs through the same intravenous line.
- Preparation
Visually inspect drug product for particulate matter and discoloration prior to administration whenever solution and container permit. Atezolizumab is a colorless to slightly yellow solution. Discard the vial if the solution is cloudy, discolored, or visible particles are observed. Do not shake the vial.
Prepare the solution for infusion as follows:
- Withdraw 20 mL of Atezolizumab from the vial.
- Dilute into a 250 mL polyvinyl chloride (PVC), polyethylene (PE), or polyolefin (PO) infusion bag containing 0.9% Sodium Chloride Injection, USP.
- Dilute with 0.9% Sodium Chloride Injection only.
- Mix diluted solution by gentle inversion. Do not shake.
- Discard used or empty vials of Atezolizumab.
Monitoring
There is limited information regarding Atezolizumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Atezolizumab and IV administrations.
Overdosage
There is no information on overdose with Atezolizumab.
Pharmacology
Atezolizumab?
| |
| Therapeutic monoclonal antibody | |
| Source | zu |
| Target | PD-L1 |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Synonyms | MPDL3280A |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
PD-L1 may be expressed on tumor cells and/or tumor-infiltrating immune cells and can contribute to the inhibition of the anti-tumor immune response in the tumor microenvironment. Binding of PD-L1 to the PD-1 and B7.1 receptors found on T cells and antigen presenting cells suppresses cytotoxic T-cell activity, T-cell proliferation and cytokine production.
Atezolizumab is a monoclonal antibody that binds to PD-L1 and blocks its interactions with both PD-1 and B7.1 receptors. This releases the PD-L1/PD-1 mediated inhibition of the immune response, including activation of the anti-tumor immune response without inducing antibody-dependent cellular cytotoxicity. In syngeneic mouse tumor models, blocking PD-L1 activity resulted in decreased tumor growth.
Structure
There is limited information regarding Atezolizumab Structure in the drug label.
Pharmacodynamics
There is limited information regarding Atezolizumab Pharmacodynamics in the drug label.
Pharmacokinetics
Patients' exposures to Atezolizumab increased dose proportionally over the dose range of 1 mg/kg to 20 mg/kg, including the fixed dose 1200 mg administered every 3 weeks. Based on a population analysis that included 472 patients in the dose range, the typical population clearance was 0.20 L/day, volume of distribution at steady state was 6.9 L, and the terminal half-life was 27 days. The population PK analysis suggests steady state is obtained after 6 to 9 weeks (2 to 3 cycles) of repeated dosing. The systemic accumulation in area under the curve (AUC), maximum concentration (Cmax) and trough concentration (Cmin) was 1.91, 1.46 and 2.75-fold, respectively. In a posthoc analysis, Atezolizumab clearance was found to decrease over time, with a mean maximal reduction (% coefficient of variation [CV%]) from baseline value of approximately 17.1% (40.6%). However, the decrease in CL was not considered clinically relevant.
- Specific Populations
Age (21–89 years), body weight, gender, positive anti-therapeutic antibody (ATA) status, albumin levels, tumor burden, region or race, mild or moderate renal impairment (estimated glomerular filtration rate (eGFR) 30 to 89 mL/min/1.73 m2), mild hepatic impairment (bilirubin ≤ ULN and AST > ULN or bilirubin < 1.0 to 1.5 × ULN and any AST), level of PD-L1 expression, or ECOG status had no clinically significant effect on the systemic exposure of Atezolizumab.
The effect of severe renal impairment (eGFR 15 to 29 mL/min/1.73 m2) or moderate or severe hepatic impairment (bilirubin > ULN and AST > ULN or bilirubin ≥ 1.0 to 1.5 × ULN and any AST) on the pharmacokinetics of Atezolizumab is unknown.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to test the potential of Atezolizumab for carcinogenicity or genotoxicity.
Animal fertility studies have not been conducted with Atezolizumab; however, an assessment of the male and female reproductive organs was included in a 26-week, repeat-dose toxicity study in cynomolgus monkeys. Weekly administration of Atezolizumab to female monkeys at the highest dose tested caused an irregular menstrual cycle pattern and a lack of newly formed corpora lutea in the ovaries. This effect occurred at an estimated AUC approximately 6 times the AUC in patients receiving the recommended dose and was reversible. There was no effect on the male monkey reproductive organs.
Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-L1/PD-1 signaling increased the severity of some infections and enhanced inflammatory responses. M. tuberculosis-infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-L1 and PD-1 knockout mice and mice receiving PD-L1 blocking antibody have also shown decreased survival following infection with lymphocytic choriomeningitis virus.
Clinical Studies
Atezolizumab was investigated in Study 1, a multicenter, open-label, two-cohort trial that included patients with locally advanced or metastatic urothelial carcinoma. In Cohort 2 of Study 1, 310 patients with locally advanced or metastatic urothelial carcinoma who had disease progression during or following a platinum-containing chemotherapy regimen or who had disease progression within 12 months of treatment with a platinum-containing neoadjuvant or adjuvant chemotherapy regimen were treated with Atezolizumab. This study excluded patients who had: a history of autoimmune disease, active or corticosteroid-dependent brain metastases, administration of a live attenuated vaccine within 28 days prior to enrollment, or administration of systemic immunostimulatory agents or systemic immunosuppressive medications. Patients received an intravenous infusion of 1200 mg of Atezolizumab every 3 weeks until unacceptable toxicity or either radiographic or clinical progression. Tumor response assessments were conducted every 9 weeks for the first 54 weeks and every 12 weeks thereafter. Major efficacy outcome measures included confirmed objective response rate (ORR) as assessed by independent review facility (IRF) using Response Evaluation Criteria in Solid Tumors (RECIST v1.1) and duration of response (DOR).
In this cohort, the median age was 66 years, 78% were male, 91% of patients were Caucasian. Twenty-six percent had non-bladder urothelial carcinoma and 78% of patients had visceral metastases. Sixty-two percent of patients had an ECOG score of 1 and 35% of patients had a baseline creatinine clearance of < 60 mL/min. Nineteen percent of patients had disease progression following prior platinum-containing neoadjuvant or adjuvant chemotherapy. Forty-one percent of patients had received ≥ 2 prior systemic regimens in the metastatic setting. Seventy-three percent of patients received prior cisplatin, 26% had prior carboplatin, and 1% were treated with other platinum-based regimens.
Tumor specimens were evaluated prospectively using the VENTANA PD-L1 (SP142) Assay at a central laboratory and the results were used to define subgroups for pre-specified analyses. Of the 310 patients, 32% were classified as having PD-L1 expression of ≥ 5% (defined as PD-L1 stained tumor-infiltrating immune cells [IC] covering ≥ 5% of the tumor area). The remaining, 68% of patients, were classified as having PD-L1 expression of <5% (PD-L1 stained tumor-infiltrating IC covering < 5% of the tumor area).
Confirmed ORR in all patients and the two PD-L1 subgroups are summarized in Table 5. The median follow-up time for this cohort was 14.4 months. In 59 patients with disease progression following neoadjuvant or adjuvant therapy, the ORR was 22.0% (95% CI: 12.3%, 34.7%).
- Table 5: Summary of Efficacy from Cohort 2 of Study 1

- Previously Treated Metastatic NSCLC
The efficacy of Atezolizumab was investigated in two multi-center, international, randomized, open-label trials in patients with metastatic NSCLC who progressed during or following a platinum-containing regimen. Study 2 was a trial in 1225 patients with the primary analysis population consisting of the first 850 randomized patients and Study 3 was a trial in 287 patients. In both studies, eligible patients were stratified by PD-L1 expression status in tumor-infiltrating immune cells (IC), by the number of prior chemotherapy regimens, and by histology. Patients were randomized (1:1) to receive either Atezolizumab administered intravenously at 1200 mg every 3 weeks until unacceptable toxicity or either radiographic or clinical progression or docetaxel administered intravenously at 75 mg/m2 every 3 weeks until unacceptable toxicity or disease progression. These studies excluded patients who had: a history of autoimmune disease, had active or corticosteroid-dependent brain metastases, administration of a live attenuated vaccine within 28 days prior to enrollment, administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medications within 2 weeks prior to enrollment. Tumor assessments were conducted every 6 weeks for the first 36 weeks, and every 9 weeks thereafter. In Study 2, tumor specimens were evaluated prospectively for PD-L1 expression on tumor cells (TC) and IC using the VENTANA PD-L1 (SP142) Assay and the results were used to define the PD-L1 expression subgroups for the analyses described below.
In Study 2, among patients in the primary analysis population, the median age was 64 years (range: 33 to 85), and 61% of patients were male. The majority of patients were white (70%). Approximately three-fourths of patients had non-squamous disease (74%), 10% had known EGFR mutation, 0.2% had known ALK rearrangements, and most patients were current or previous smokers (82%). Baseline ECOG performance status was 0 (37%) or 1 (63%). Seventy five percent of patients received only one prior platinum-based therapeutic regimen. In Study 3, the median age was 62 years (range: 36 to 84), and 59% of patients were male. The majority of patients were white (79%). Approximately two-thirds of patients had non-squamous disease (66%), 7% had known EGFR mutation, 1% had ALK rearrangements, and most patients were current or previous smokers (80%). Baseline ECOG performance status was 0 (33%) or 1 (67%). Approximately two-thirds of patients received only one prior platinum-based therapeutic regimen.
The major efficacy outcome measure of Study 2 was overall survival (OS) in the primary analysis population (first 850 randomized patients). The major efficacy outcome measure of Study 3 was overall survival (OS). Other efficacy outcome measures for Study 3 included investigator-assessed objective response rates and duration of response per RECIST v1.1. The results of Study 2 with a median follow up of 21 months are presented in Table 6 and Figure 1.
- Table 6: Efficacy Results in the Primary Analysis Population from Study 2

TECENTRIQ: Atezolizumab's Brand name
- Figure 1: Kaplan-Meier Plot of Overall Survival in the Primary Analysis Population in Study 2

TECENTRIQ: Atezolizumab's Brand name
Tumor specimens were evaluated prospectively using the VENTANA PD-L1 (SP142) Assay at a central laboratory and the results were used to define the PD-L1 expression subgroups for pre-specified analyses. Of the 850 patients, 16% were classified as having high PD-L1 expression, defined as having PD-L1 expression on ≥ 50% of TC or ≥ 10% of IC. In an exploratory efficacy subgroup analysis of OS based on PD-L1 expression, the hazard ratio was 0.41 (95% CI: 0.27, 0.64) in the high PD-L1 expression subgroup and 0.82 (95% CI: 0.68, 0.98) in patients who did not have high PD-L1 expression.
Results of an updated survival analysis in Study 3 with a median follow-up of 22 months are provided for all randomized patients (Table 7 and Figure 2).
- Table 7: Efficacy Results from Study 3
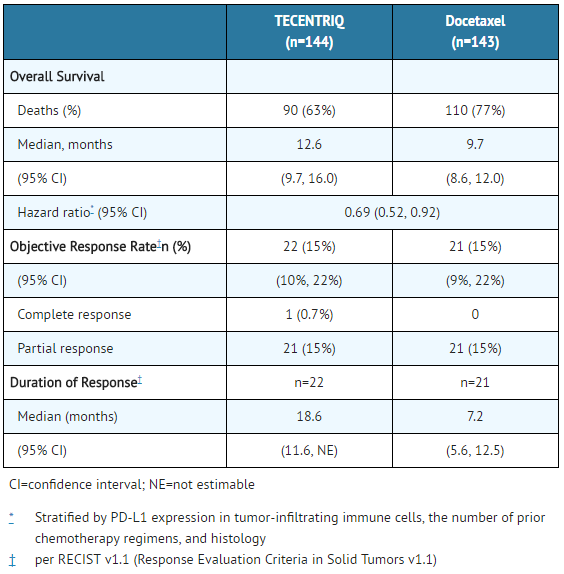
TECENTRIQ: Atezolizumab's Brand name
- Figure 2: Kaplan-Meier Plot of updated Overall Survival in Study 3

TECENTRIQ: Atezolizumab's Brand name
How Supplied
Atezolizumab injection is a sterile, preservative-free, and colorless to slightly yellow solution for intravenous infusion supplied as a carton containing one 1200 mg/20 mL single-dose vial (NDC 50242-917-01).
Storage
- This product does not contain a preservative.
- Administer immediately once prepared. If diluted Atezolizumab infusion solution is not used immediately, it can be stored either:
- At room temperature for no more than 6 hours from the time of preparation. This includes room temperature storage of the infusion in the infusion bag and time for administration for infusion.
- Under refrigeration at 2°C–8°C (36°F–46°F) for no more than 24 hours.
- Do not freeze.
- Do not shake.
Images
Drug Images
{{#ask: Page Name::Atezolizumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Atezolizumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling.
Inform patients of the risk of immune-related adverse reactions that may require corticosteroid treatment and interruption or discontinuation of Atezolizumab, including:
- Pneumonitis: Advise patients to contact their healthcare provider immediately for any new or worsening cough, chest pain, or shortness of breath.
- Hepatitis: Advise patients to contact their healthcare provider immediately for jaundice, severe nausea or vomiting, pain on the right side of abdomen, lethargy, or easy bruising or bleeding.
- Colitis: Advise patients to contact their healthcare provider immediately for diarrhea or severe abdominal pain.
- Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of hypophysitis, hyperthyroidism, hypothyroidism, adrenal insufficiency, or type 1 diabetes mellitus, including diabetic ketoacidosis.
- Meningoencephalitis, myasthenic syndrome/myasthenia gravis, and Guillain-Barré syndrome: Advise patients to contact their healthcare provider immediately for signs or symptoms of meningitis, myasthenic syndrome/myasthenia gravis, or Guillain-Barré syndrome.
- Ocular Inflammatory Toxicity: Advise patients to contact their healthcare provider immediately for signs or symptoms of ocular inflammatory toxicity.
- Pancreatitis: Advise patients to contact their healthcare provider immediately for signs and symptoms of pancreatitis.
- Infection: Advise patients to contact their healthcare provider immediately for signs or symptoms of infection.
- Infusion-Related Reactions: Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions.
- Rash: Advise patients to contact their healthcare provider immediately for signs or symptoms of rash.
Embryo-Fetal Toxicity
Advise female patients that Atezolizumab can cause fetal harm. Instruct females of reproductive potential to use effective contraception during treatment and for at least 5 months after the last dose of Atezolizumab.
Lactation
Advise female patients not to breastfeed while taking Atezolizumab and for at least 5 months after the last dose.
Precautions with Alcohol
Alcohol-Atezolizumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
TECENTRIQ™
Look-Alike Drug Names
There is limited information regarding Atezolizumab Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.