Asplenia pathophysiology
|
Asplenia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Asplenia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Asplenia pathophysiology |
|
Risk calculators and risk factors for Asplenia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Kalpana Giri, MBBS[2]
Overview
Asplenia can refer to an anatomic absence of the spleen or functional asplenia secondary to a variety of disease states. The absence of a spleen is a well-known risk factor for severe bacterial infections, especially due to encapsulated bacteria. The primary physiologic role of spleen is the filtration and processing of senescent blood cells, predominantly red blood cells and immunologically helps protect against encapsulated microorganisms and response to infectious pathogens. The spleen plays integral roles in the immune system and reticuloendothelial systems.
Pathophysiology
Physiology
The spleen consists of three functional inter-related compartments: red pulp, white pulp, marginal zone. The red pulp is a sponge-like structure filled with blood flowing through sinuses and cords functions as a filter for blood elements.[1] The white pulp consists primarily of lymphatic tissue creating structures called germinal centers which contain lymphocytes (activated B-lymphocytes among others), macrophages, and dendritic cells. They are situated in direct contact with splenic arterioles, branches of the splenic artery. Another region of the white pulp is that the periarteriolar lymphatic sheath, which consists of nodules containing mostly B lymphocytes. The marginal zone surrounds the white pulp and consists of blood vessels, macrophages, and specialized B cells.[2] The primary physiologic role of spleen is the filtration and processing of senescent blood cells, predominantly red blood cells and immunologically helps protect against encapsulated microorganisms and response to infectious pathogens. It contains both hematopoietic and lymphopoietic elements, which provides a basis for extramedullary hematopoiesis when necessary.
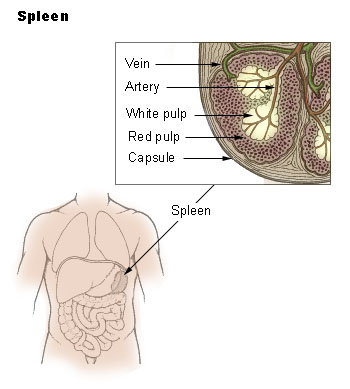 |
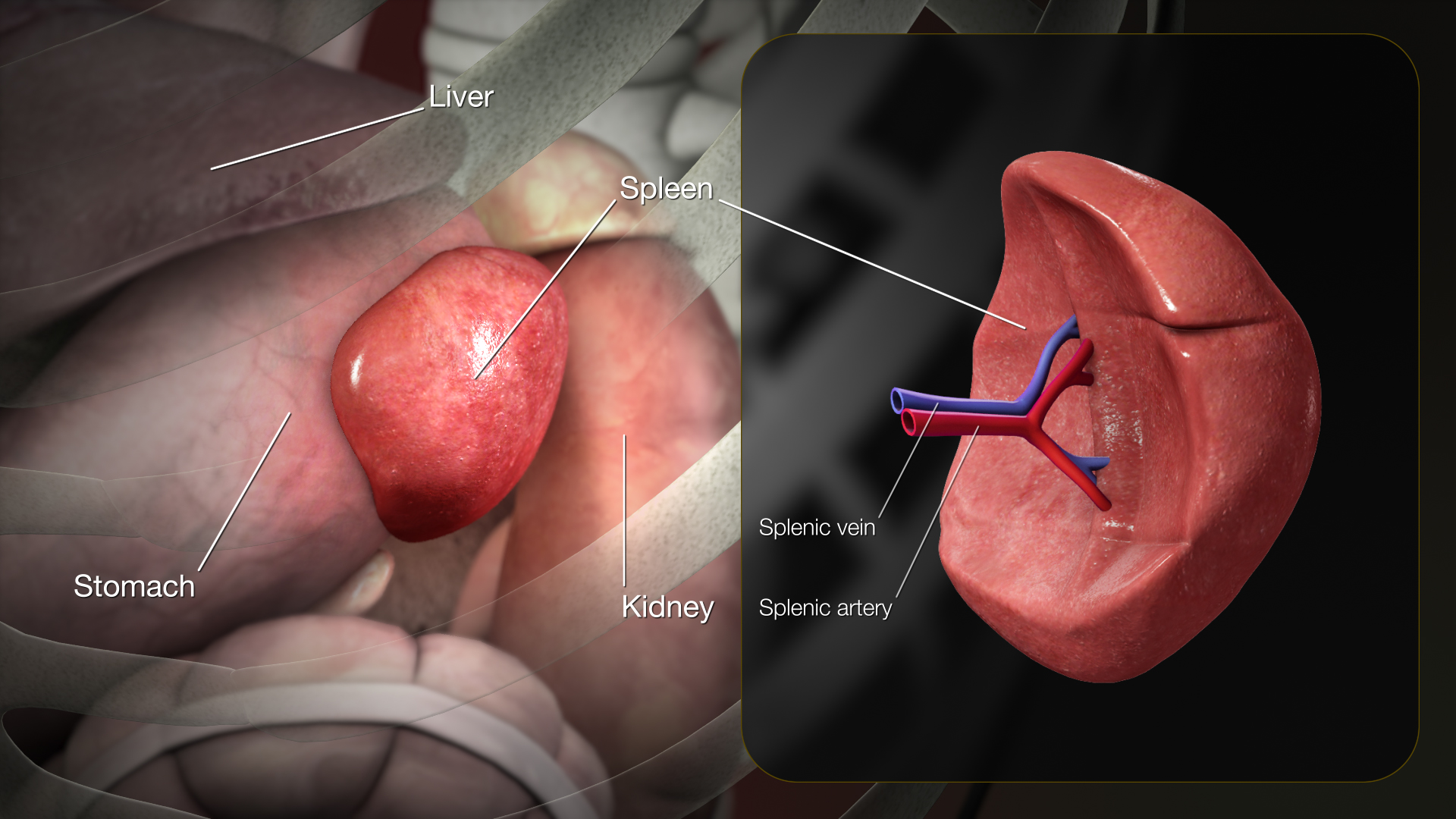 |
 |
Pathology
The spleen plays integral roles in the immune system and reticuloendothelial systems. It also modulates the inflammatory and coagulation cascades.[3] It is understood that Asplenia is a variety of clinical settings, and it can refer to an anatomic absence of the spleen or functional asplenia secondary to a variety of disease states. The risk of death from septicaemia is 200 times higher in asplenic patients than the individual with a spleen.[4] The absence of a spleen is a well-known risk factor for severe bacterial infections, especially due to encapsulated bacteria. The spleen contains 2 types of tissues: white pulp and red pulp. The white pulp is rich in T-cell lymphocytes, naïve B-cell lymphocytes, and macrophages. The antigen-presenting cells (APC) can enter the white pulp and activate T cells, which in turn activate naïve B cells and differentiate into plasma cells that generate immunoglobulin M antibodies followed by immunoglobulin G antibodies. B cells can also act as antigen-presenting cells and has a phagocytic function to help opsonize encapsulated bacteria. About half of the total B cells in the blood express the memory marker CD27 and carry somatic mutations, and are therefore thought to be memory B cells. There are two types of memory B cells in human beings: switched memory B cells and IgM memory B cells. Switched memory B cells, which are the final product of germinal center reactions, produce high-affinity antibodies and have a protective function against infection. IgM memory B cells, need the spleen for their survival and generation and have the ability to produce natural antibodies. They also produce antibodies against Streptococcus pneumonia, Neisseria meningitidis, and Haemophilus influenzae type b. They can initiate T-cell-independent immune responses on infection or vaccination with capsular polysaccharide antigens.[1] The red pulp has macrophages and is responsible for filtering damaged, older red blood cells as well as phagocytosing opsonized bacteria. Due to this role of removing damaged erythrocytes, the spleen also plays an important role in the defense against intraerythrocytic parasitic infections such as malaria and Babesia.[5] About 30% of platelets are sequestrated in the splenic tissue, spleen is the main site of storage of circulating platelels. [2]
Functional asplenia is associated with sickle cell anemia, hemoglobin sickle cell disease, and sickle cell hemoglobin β thalassemia. Patient with these hemoglobinopathies starts losing a splenic function, where the spleen is initially enlarged due to excessive red cell entrapment results in atrophy and degeneration in advanced disease. This atrophy is called autosplenectomy and may be consequent] to multiple acute episodes of entrapment of massive red cell volumes in the splenic tissue, followed by splenic infarctions. Functional hyposplenism associated with celiac disease and inflammatory bowel disease leads to spleen’s reticuloendothelial atrophy due to loss of lymphocytes through the inflamed enteric mucosa. Hyposplenism in autoimmune disorders one of the major mechanisms could be reticuloendothelial block due to circulating [[immune complexes]. In hematologic and neoplastic disorders, it is probably due to splenic tissue infiltration by tumor cells or due to vascular occlusion. Hyposplenism in hepatic disorders, might be caused by disruption of normal hepatic microcirculation due to portal hypertension. In acute or chronic alcohol consumption, direct toxic effect of alcohol is implied in all disorders.[2]
Genetics
Genes involved in the pathogenesis of Isolatd congenital asplenia include: Mutations in RPSA exons can affect the translated or untranslated regions and can underlie Isolatd congenital asplenia(ICA) with complete or incomplete penetrance.[6]
References
- ↑ 1.0 1.1 Di Sabatino A, Carsetti R, Corazza GR (2011) Post-splenectomy and hyposplenic states. Lancet 378 (9785):86-97. DOI:10.1016/S0140-6736(10)61493-6 PMID: 21474172
- ↑ 2.0 2.1 2.2 Kirkineska L, Perifanis V, Vasiliadis T (2014). dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25125944 "Functional hyposplenism" Check
|url=value (help). Hippokratia. 18 (1): 7–11. PMC 4103047. PMID 25125944. - ↑ Long B, Koyfman A, Gottlieb M (2021). "Complications in the adult asplenic patient: A review for the emergency clinician". Am J Emerg Med. 44: 452–457. doi:10.1016/j.ajem.2020.03.049. PMID 32247651 Check
|pmid=value (help). - ↑ Erdem SB, Genel F, Erdur B, Ozbek E, Gulez N, Mese T (2015). "Asplenia in children with congenital heart disease as a cause of poor outcome". Cent Eur J Immunol. 40 (2): 266–9. doi:10.5114/ceji.2015.52841. PMC 4637402. PMID 26557043.
- ↑ Lee GM (2020). "Preventing infections in children and adults with asplenia". Hematology Am Soc Hematol Educ Program. 2020 (1): 328–335. doi:10.1182/hematology.2020000117. PMC 7727556 Check
|pmc=value (help). PMID 33275684 Check|pmid=value (help). - ↑ Bolze A, Boisson B, Bosch B, Antipenko A, Bouaziz M, Sackstein P; et al. (2018). "Incomplete penetrance for isolated congenital asplenia in humans with mutations in translated and untranslated RPSA exons". Proc Natl Acad Sci U S A. 115 (34): E8007–E8016. doi:10.1073/pnas.1805437115. PMC 6112730. PMID 30072435.