Aminoglutethimide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Aminoglutethimide is an aromatase inhibitor that is FDA approved for the treatment of suppression of adrenal function in selected patients with Cushing’s syndrome. Common adverse reactions include drowsiness , morbilliform skin rash , nausea and anorexia , and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Cushing’s syndrome
- Aminoglutethimide is indicated for the suppression of adrenal function in selected patients with Cushing’s syndrome. Morning levels of plasma cortisol in patients with adrenal carcinoma and ectopic ACTH producing tumors were reduced on the average to about one half of the pretreatment levels, and in patients with adrenal hyperplasia to about two thirds of the pretreatment levels, during 1-3 months of therapy with Aminoglutethimide. Data available from the few patients with adrenal adenoma suggest similar reductions in plasma cortisol levels. Measurements of plasma cortisol showed reductions to at least 50% of baseline or to normal levels in one third or more of the patients studied, depending on diagnostic groups and time of measurement.
- Because Aminoglutethimide does not affect the underlying disease process, it is used primarily as an interim measure until more definitive therapy such as surgery can be undertaken or in cases where such therapy is not appropriate. Only small numbers of patients have been treated for longer than 3 months. A decreased effect or “escape phenomenon” seems to occur more frequently in patients with pituitary dependent Cushing’s syndrome, probably because of increasing ACTH levels in response to decreasing glucocorticoid levels.
- Aminoglutethimide should be used only in those patients who are responsive to treatment.
Dosage and Administration
- Treatment should be instituted in a hospital until a stable dosage regimen is achieved. Therapy should be initiated with 250 mg orally four times daily, preferably at 6-hour intervals. Adrenocortical response should be followed by careful monitoring of plasma cortisol levels until the desired level of suppression is achieved. If the level of cortisol suppression is inadequate, the dosage may be increased in increments of 250 mg daily at intervals of 1-2 weeks to a total daily dose of 2 g. Dose reduction or temporary discontinuation of therapy may be required in the event of adverse effects, including extreme drowsiness, severe skin rash, or excessively low cortisol levels. If a skin rash persists for longer than 5-8 days or becomes severe, the drug should be discontinued. It may be possible to reinstate therapy at a lower dosage following the disappearance of a mild or moderate rash. Mineralocorticoid replacement (e.g., fludrocortisone) may be necessary. If glucocorticoid replacement therapy is needed, 20-30 mg of hydrocortisone orally in the morning will replace endogenous secretion.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aminoglutethimide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aminoglutethimide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Aminoglutethimide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aminoglutethimide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aminoglutethimide in pediatric patients.
Contraindications
- Aminoglutethimide is contraindicated in those patients with serious forms, and/or more severe manifestations, of hypersensitivity to glutethimide or aminoglutethimide.
Warnings
- Aminoglutethimide may cause adrenocortical hypofunction, especially under conditions of stress, such as surgery, trauma, or acute illness. Patients should be carefully monitored and given hydrocortisone and mineralocorticoid supplements as indicated. Dexamethasone should not be used.
- Aminoglutethimide also may suppress aldosterone production by the adrenal cortex and may cause orthostatic or persistent hypotension. The blood pressure should be monitored in all patients at appropriate intervals. Patients should be advised of the possible occurrence of weakness and dizziness as symptoms of hypotension, and of measures to be taken should they occur.
Precautions
General
- This drug should be administered only by physicians familiar with its use and hazards. Therapy should be initiated in a hospital.
Adverse Reactions
Clinical Trials Experience
- Untoward effects have been reported in about 2 out of 3 patients with Cushing’s syndrome who were treated for 4 or more weeks with Aminoglutethimide as the only adrenocortical suppressant.
- The most frequent and reversible side effects were drowsiness (approximately 1 in 3 patients), morbilliform skin rash (1 in 6 patients), nausea and anorexia (each approximately 1 in 8 patients), and dizziness (about 1 in 20 patients). The dizziness was possibly caused by lowered vascular resistance or orthostasis. These reactions often disappear spontaneously with continued therapy.
Other Effects Observed
- Hematologic: Single instances of neutropenia, leukopenia (patient received concomitant o,p'-DDD), pancytopenia (patient received concomitant 5-fluorouracil), and agranulocytosis occurred in 4 of 27 patients with Cushing’s syndrome caused by adrenal carcinoma who were treated for at least 4 weeks. In 1 patient with adrenal hyperplasia, hemoglobin levels and hematocrit decreased during the course of treatment with Aminoglutethimide. From the earlier experience with the drug used as an anticonvulsant in 1,214 patients, transient leukopenia was the only hematologic effect and was reported once; Coombs’- negative hemolytic anemia also occurred once. In approximately 300 patients with nonadrenal malignancy, 1 in 25 showed some degree of anemia, and 1 in 150 developed pancytopenia during treatment with Aminoglutethimide.
- Endocrine: Adrenal insufficiency occurred in about 1 in 30 patients with Cushing’s syndrome who were treated with Aminoglutethimide for 4 or more weeks. This insufficiency tended to involve glucocorticoids as well as mineralocorticoids. Hypothyroidism is occasionally associated with thyroid enlargement and may be detected or confirmed by measuring plasma levels of the thyroid hormone. Masculinization and hirsutism have occasionally occurred in females, as has precocious sexual development in males.
- Central Nervous System: Headache was reported in about 1 in 20 patients.
- Cardiovascular: Hypotension, occasionally orthostatic, occurred in 1 in 30 patients receiving Aminoglutethimide. Tachycardia occurred in 1 in 40 patients.
- Gastrointestinal and Liver: Vomiting occurred in 1 in 30 patients. Isolated instances of abnormal findings on liver function tests were reported. Suspected hepatotoxicity occurred in less than 1 in 1000 patients.
- Skin: In addition to rash (1 in 6 patients, and often reversible with continued therapy), pruritus was reported in 1 in 20 patients. These may be allergic or hypersensitive reactions. Urticaria has occurred rarely.
- Miscellaneous: Fever was reported in several patients who were treated with Aminoglutethimide for less than 4 weeks; some of these patients also received other drugs. Myalgia occurred in 1 in 30 patients. Pulmonary hypersensitivity, including allergic alveolitis and interstitial alveolar infiltrates, has occurred rarely
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Aminoglutethimide in the drug label.
Drug Interactions
- Aminoglutethimide accelerates the metabolism of dexamethasone; therefore, if glucocorticoid replacement is needed, hydrocortisone should be prescribed.
- Aminoglutethimide diminishes the effect of coumarin and warfarin.
Use in Specific Populations
Pregnancy
- Cytadren can cause fetal harm when administered to a pregnant woman. In the earlier experience with the drug in about 5000 patients, two cases of pseudohermaphroditism were reported in female infants whose mothers were treated with Cytadren and concomitant anticonvulsants. Normal pregnancies have also occurred in patients treated with Cytadren.
- When administered to rats at doses 1/2 and 1 1/4 times the maximum daily human dose, Cytadren caused a decrease in fetal implantation, an increase in fetal deaths, and a variety of teratogenic effects. The compound also caused pseudohermaphroditism in rats treated with approximately 3 times the maximum daily human dose. If this drug must be used during pregnancy, or if the patient becomes pregnant while taking the drug, the patient should be apprised of the potential hazard to the fetus.
There is no FDA guidance on usage of Aminoglutethimide in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aminoglutethimide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aminoglutethimide during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Aminoglutethimide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established
Geriatic Use
- Clinical studies of Aminoglutethimide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Spontaneous post-marketing adverse event reports and reports from the published literature have not identified obvious differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Aminoglutethimide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aminoglutethimide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Aminoglutethimide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Aminoglutethimide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aminoglutethimide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aminoglutethimide in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Aminoglutethimide Administration in the drug label.
Monitoring
- Hypothyroidism may occur in association with Aminoglutethimide; hence, appropriate clinical observations should be made and laboratory studies of thyroid function performed as indicated. Supplementary thyroid hormone may be required.
- Hematologic abnormalities in patients receiving Aminoglutethimide have been reported . Therefore, baseline hematologic studies should be performed, followed by periodic hematologic evaluation.
- Since elevations in SGOT, alkaline phosphatase, and bilirubin have been reported, appropriate clinical observations and regular laboratory studies should be performed before and during therapy. Serum electrolyte levels should be determined periodically.
- Since elevations in SGOT, alkaline phosphatase, and bilirubin have been reported, appropriate clinical observations and regular laboratory studies should be performed before and during therapy.
- Serum electrolyte levels should be determined periodically
IV Compatibility
There is limited information regarding IV Compatibility of Aminoglutethimide in the drug label.
Overdosage
Acute Toxicity
- No deaths due to overdosage with Aminoglutethimide have been reported.
- The highest known doses that have been survived are 7 g (33-year-old woman), 7.5-10 g (16- year-old girl), and 10 g (10-year-old boy).
- Oral LD50’s (mg/kg): rats, 1800; dogs, >100. Intravenous LD50’s (mg/kg): rats, 156; dogs, >100.
Signs and symptoms
- An acute overdose with Aminoglutethimide may reduce the production of steroids in the adrenal cortex to a degree that is clinically relevant. The following manifestations may be expected:
- Respiratory Function: Respiratory depression, hypoventilation. Cardiovascular System: Hypotension, hypovolemic shock due to dehydration.
- Central Nervous System/Muscles: Somnolence, lethargy, coma, ataxia, dizziness, fatigue. (Extreme weakness has been reported with divided doses of 3 g daily.) :*Gastrointestinal System: Nausea, vomiting.
- Renal Function: Loss of sodium and water.
- Laboratory Findings: Hyponatremia, hypochloremia, hyperkalemia, hypoglycemia.
- The signs and symptoms of acute overdosage with Aminoglutethimide may be aggravated or modified if alcohol, hypnotics, tranquilizers, or tricyclic antidepressants have been taken at the same time.
Treatment
- Symptomatic treatment of overdosage is recommended.
- Since aminoglutethimide and glutethimide are chemically related, measures that have been used in successfully removing glutethimide from the body might be useful in removing aminoglutethimide.
- Gastric lavage and unspecified supportive treatment have been employed. Full consciousness following deep coma was regained 40 hours or less after ingestion of 3 or 4 g without lavage. No evidence of hematologic, renal, or hepatic effects was subsequently found.
- Close monitoring should be provided, and appropriate measures taken to support vital functions, if necessary.
- If deficiency of circulating glucocorticoid develops, an intravenous infusion of a soluble hydrocortisone preparation (100 mg of hydrocortisone sodium succinate in 500 mL of isotonic sodium chloride solution) and 50 mL of 40% glucose solution should be given within 3 hours. After the initial infusion is completed, an intravenous administration of hydrocortisone, 10 mg per hour, should be continued until the patient is able to take oral cortisone.
- If hypovolemia or hypotension occurs, an intravenous administration of norepinephrine, 10 mg, in 500 mL of isotonic sodium chloride should be administered according to the patient’s needs and response. After rehydration, 500 mL of plasma or blood should be given for maintenance of sufficient circulatory volume. *Dialysis may be considered in severe intoxication.
Pharmacology
There is limited information regarding Aminoglutethimide Pharmacology in the drug label.
Mechanism of Action
- Aminoglutethimide inhibits the enzymatic conversion of cholesterol to ∆5 -pregnenolone, resulting in a decrease in the production of adrenal glucocorticoids, mineralocorticoids, estrogens, and androgens.
- Aminoglutethimide blocks several other steps in steroid synthesis, including the C-11, C-18, and C-21 hydroxylations and the hydroxylations required for the aromatization of androgens to estrogens, mediated through the binding of Aminoglutethimide to cytochrome P-450 complexes.
- A decrease in adrenal secretion of cortisol is followed by an increased secretion of pituitary adrenocorticotropic hormone (ACTH), which will overcome the blockade of adrenocortical steroid synthesis by Aminoglutethimide. The compensatory increase in ACTH secretion can be suppressed by the simultaneous administration of hydrocortisone. Since Aminoglutethimide increases the rate of metabolism of dexamethasone but not that of hydrocortisone, the latter is preferred as the adrenal glucocorticoid replacement.
- Although Aminoglutethimide inhibits the synthesis of thyroxine by the thyroid gland, the compensatory increase in thyroid-stimulating hormone (TSH) is frequently of sufficient magnitude to overcome the inhibition of thyroid synthesis due to Aminoglutethimide. In spite of an increase in TSH, Aminoglutethimide has not been associated with increased prolactin secretion.
Note: Aminoglutethimide was marketed previously as an anticonvulsant but was withdrawn from marketing for that indication in 1966 because of the effects on the adrenal gland.
Structure
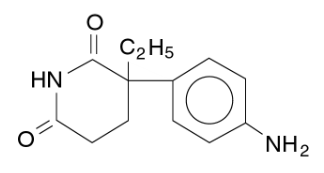
- Aminoglutethimide, aminoglutethimide tablets USP, is an inhibitor of adrenocortical steroid synthesis, available as 250-mg tablets for oral administration. Its chemical name is 3-(4-aminophenyl)-3-ethyl-2,6- piperidinedione, and its structural formula is
- Aminoglutethimide USP is a fine, white or creamy white, crystalline powder. It is very slightly soluble in water, and readily soluble in most organic solvents. It forms water- soluble salts with strong acids. Its molecular weight is 232.28. Inactive Ingredients. Cellulose compounds, colloidal silicon dioxide, starch, stearic acid, and talc.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Aminoglutethimide in the drug label.
Pharmacokinetics
- Aminoglutethimide is rapidly and completely absorbed after oral administration. In 6 healthy male volunteers, maximum plasma levels of Aminoglutethimide averaged 5.9 µg/mL at a median of 1.5 hours after ingestion of two 250-mg tablets. The bioavailability of tablets is equivalent to equal doses given as a solution. After ingestion of a single oral dose, 34%-54% is excreted in the urine as unchanged drug during the first 48 hours, and an additional fraction as the N-acetyl derivative.
- The half-life of Aminoglutethimide in normal volunteers given single oral doses averaged 12.5 ± 1.6 hours.
- Upon withdrawal of therapy with Aminoglutethimide, the ability of the adrenal glands to synthesize steroid returns, usually within 72 hours.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- A 2-year carcinogenicity study of Aminoglutethimide conducted in rats at doses of 10-60 mg/kg/day (approximately 0.04 to 0.2 times the maximum daily therapeutic dose based on surface area, mg/m2 ) revealed a highly statistically significant dose-related trend in the incidence of benign and malignant neoplasms of the adrenal cortex and thyroid follicular cells in both sexes. A borderline statistically significant increase (0.05 level) in ovarian tubular adenomas was observed at 60 mg/kg/day. Urinary bladder papillomas also showed a statistically significant dose-related trend in males.
- Aminoglutethimide affects fertility in female rats . The relevance of these findings to humans is not known.
Clinical Studies
CLINICAL STUDIES IN CHILDREN
- Clinical investigations included 9 patients aged 2 1/2 to 16 years; 4 of these were aged 10 or less. Seven of the patients received other therapies (drugs or irradiation) either with Aminoglutethimide or within a short period before initiation of therapy with Aminoglutethimide. Diagnoses included 5 patients with adrenal carcinoma, 3 with adrenal hyperplasia, and 1 with ectopic ACTH-producing tumor. Duration of treatment ranged from 3 days to 6 1/2 months. Dosages ranged from 0.375 g to 1.5 g daily. In general, smaller doses were used for younger patients; for example, a 2 1/2-year- old received 0.5-0.75 g daily, a 3 1/2-year-old received 0.5 g daily, and all others over 10 years of age received 0.75-1.5 g daily. Results are difficult to evaluate because of the concomitant therapy, duration of therapy, or inadequate laboratory documentation. Most patients did show decreases in plasma or urinary steroids at some time during treatment, but these may have been due to other therapeutic modalities or their combinations.
How Supplied
- Tablets 250 mg — white, round, scored into quarters (imprinted CIBA 24)
- Bottles of 100 ........................................................................... NDC 0083-0024-30
- Protect from light.
Storage
- Do not store above 30ºC (86ºF).
- Dispense in tight, light-resistant container (USP)
Images
Drug Images
{{#ask: Page Name::Aminoglutethimide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Aminoglutethimide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be warned that drowsiness may occur and that they should not drive, operate potentially dangerous machinery, or engage in other activities that may become hazardous because of decreased alertness.
- Patients should also be warned of the possibility of hypotension and its symptoms
Precautions with Alcohol
- *The effects of Aminoglutethimide may be potentiated if it is taken in combination with alcohol.
Brand Names
- Cytadren
Look-Alike Drug Names
There is limited information regarding Aminoglutethimide Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Aminoglutethimide
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}