Trimethoprim
For patient information, click here.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Trimethoprim (INN) (IPA: Template:IPA) is a bacteriostaticantibiotic mainly used in the prophylaxis and treatment of urinary tract infections. It belongs to the class ofchemotherapeutic agents known as dihydrofolate reductase inhibitors. Trimethoprim was formerly marketed byGlaxoWellcome under trade names including Proloprim, Monotrim and Triprim; but these trade names have been licensed to various generic pharmaceutical manufacturers. In clinical use it is often abbreviated TRI or TMP; its common laboratory abbreviation is W.
Category
Folate antagonist
US Brand Names
PRIMSOL®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Mechanism of Action
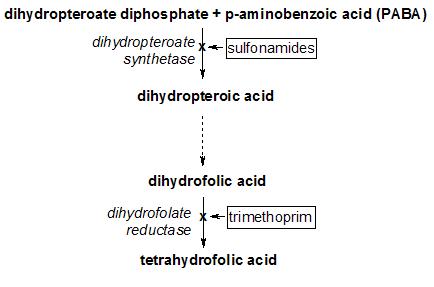
Trimethoprim acts by interfering with the action of bacterial dihydrofolate reductase, inhibiting synthesis of tetrahydrofolic acid. Tetrahydrofolic acid is an essential precursor in the de novo synthesis of the DNA nucleotide thymine. Bacteria are unable to take up folic acid from the environment (i.e. the infection host) and are thus dependent on their own de novo synthesis. Inhibition of the enzyme starves the bacteria of bases necessary for DNA replication.