Multiple myeloma pathophysiology
|
Multiple myeloma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Multiple myeloma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Multiple myeloma pathophysiology |
|
Risk calculators and risk factors for Multiple myeloma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Haytham Allaham, M.D. [2]
Overview
Multiple myeloma arises from post-germinal centre plasma cells, that are normally involved in production of human immunoglobulins.[1][2][3][4] Development of multiple myeloma is the result of multiple genetic translocations between the immunoglobulin heavy chain gene and oncogenes which lead to dysregulated multiplication of plasma cells.[2][3] On microscopic histopathological analysis, abundant eosinophilic cytoplasm, eccentrically placed nucleus, and russell bodies are characteristic findings of multiple myeloma.[5]
Pathophysiology
Pathogenesis
- Multiple myeloma arises from post-germinal centre plasma cells, that are normally involved in production of human immunoglobulins.[2][3][6]
- Malignant plasma cell infiltrates hematopoietic sites such as the red bone marrow where they interfere with the production of normal blood cells.[1][2][3][6]
Pathophysiology of Osseous Involvement
Multiple myeloma is one of the most common malignancies that creates lytic bony lesions. Other cancers that can create lytic bony lesions include renal cell carcinoma, lung cancer, breast cancer, thyroid cancer, and lymphoma. Lytic destruction has a distinct pathophysiology compared to the blastic destruction that is seen in prostate cancer. The pathophysiology of bony disease in multiple myeloma begins with stimulation of osteoclastic production and suppression of osteoblast production. The steady state of bone metabolism is shifted in the direction of bone resorption. The molecular mechanism that governs osteoclast activation in multiple myeloma involves nuclear factor kappa B (NFkB), interleukin-3 (IL-3), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-alpha), and CXCL12, also known as stromal cell-derived factor 1 (SDF1).[7]
- Distribution of multiple myeloma mirrors that of red bone marrow in older individuals, and thus multiple myeloma is mostly encountered in the axial skeleton and proximal appendicular skeleton such as:[1]
- Vertebrae (most common)
- Ribs
- Skull
- Shoulder girdle
- Pelvis
- Long bones
- Extra skeletal structures (extraosseous myeloma) (rare)
- Abnormal antibodies are produced in multiple myeloma and are deposited in various organs around the body leading to multiple complications such as nephropathy, polyneuropathy, and cardiomyopathy.[2][4]
- Production of cytokines (especially IL-6) by malignant plasma cells in multiple myeloma causes much of their localized damage, such as osteoporosis. The produced cytokines also increase angiogenesis and create a microenvironment in which the malignant plasma cells can thrive.[2][6]
- Multiple myeloma may result in renal failure due to either glomerular deposition of amyloid or tubular damage from excretion of light chains called Bence Jones proteins. This can manifest as Fanconi syndrome (type II renal tubular acidosis).[2][1][6]
- Immune deficiency occurs in multiple myeloma as the majority of the overproduced antibodies are ineffective monoclonal antibodies produced by malignant plasma cell.[2]
Genetics
- Normally the immune system keeps the proliferation of B cells and the secretion of antibodies under tight control. When chromosomes and genes are damaged, often through rearrangement, this control is lost.[2]
- Often, a promoter gene translocates to a chromosome where it stimulates overproduction of an antibody gene.[2]
- This genetic mutation results in dysregulation of the oncogene which is thought to be an important initiating event in the pathogenesis of multiple myeloma.
- The genes involved in the pathogenesis of multiple myeloma include heavy chain gene (on the chromosome 14, locus 14q32), chromosome 13, and oncogenes (often 11q13, 4p16.3, 6p21, 16q23 and 20q11).[8]
- A familial predisposition to myeloma exists. Hyperphosphorylation of the paratarg proteins, a tendency which is inherited in an autosomal dominant manner, appears to be a common mechanism in these families. This tendency is more common in African American patients with myeloma and may contribute to the higher rates of myeloma in this group.[2][9]
Gross Pathology
-
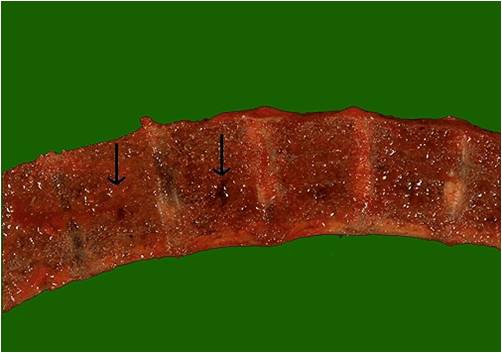
Vertebrae in multiple myeloma
(Image courtesy of Melih Aktan M.D.) -
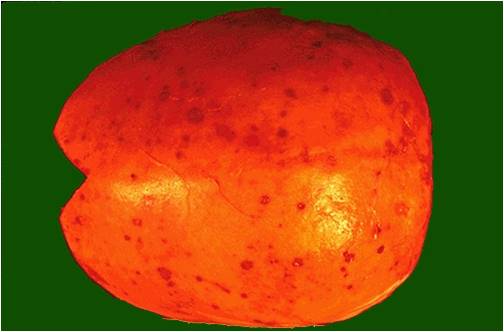
Calvarium in multiple myeloma.
(Image courtesy of Melih Aktan M.D.)
Microscopic Pathology
On microscopic histopathological analysis, multiple myeloma is characterized by the following:[5]
- Abundant eosinophilic cytoplasm
- Eccentrically placed nucleus
- Clock face morphology of the nucleus due to chromatin clumps around the edges
- Russell bodies which are eosinophilic, large (10-15 micrometres), homogenous immunoglobulin-containing inclusions
- Dutcher bodies which are PAS stain +ve intranuclear crystalline rods
- Shown below is a series of microscopic images seen in multiple myeloma:
-
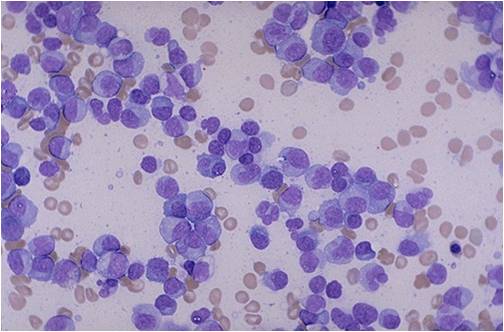
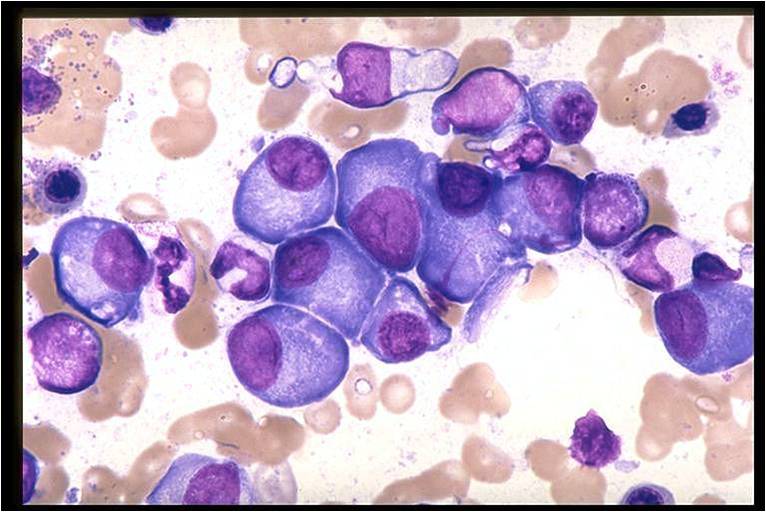
Multiple Myeloma slide showing plasma cells with large nucleus and scant cytoplasm [10]
-
Bone marrow aspiration in multiple myeloma.
(Image courtesy of Melih Aktan M.D.) -
Bone marrow biopsy in multiple myeloma.
(Image courtesy of Melih Aktan M.D.) -
Bone marrow in multiple myeloma.
(Image courtesy of Melih Aktan M.D.) -
Bone marrow in multiple myeloma.
(Image courtesy of Melih Aktan M.D.) -
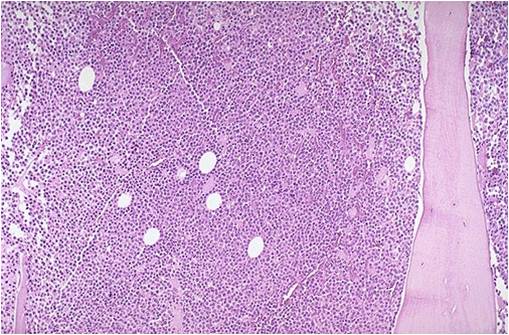
Multiple myeloma slide with intermediate magnification[5]
-
Multiple myeloma slide with high magnification[5]
-
Multiple myeloma slide with russell bodies[5]
References
- ↑ 1.0 1.1 1.2 1.3 Multiple myeloma. Radiopaedia (2015)http://radiopaedia.org/articles/multiple-myeloma-1 Accessed on September, 20th 2015
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Multiple myeloma. Wikipedia (2015)https://en.wikipedia.org/wiki/Multiple_myeloma#Pathophysiology Accessed on September, 20th 2015
- ↑ 3.0 3.1 3.2 3.3 Multiple myeloma. Medlineplus (2015)https://www.nlm.nih.gov/medlineplus/multiplemyeloma.html Accessed on September, 20th 2015
- ↑ 4.0 4.1 Multiple myeloma. National cancer institute (2015) Accessed on September, 20th 2015
- ↑ 5.0 5.1 5.2 5.3 5.4 Multiple myeloma. Librepathology (2015)http://www.wikidoc.org/index.php?title=Multiple_myeloma_pathophysiology&action=edit§ion=1 Accessed on September, 20th 2015
- ↑ 6.0 6.1 6.2 6.3 What is multiple myeloma. Canadian Cancer Society (2015) http://www.cancer.ca/en/cancer-information/cancer-type/multiple-myeloma/multiple-myeloma/?region=mb Accessed on September, 20th 2015
- ↑ Yaccoby S (2010). "Advances in the understanding of myeloma bone disease and tumour growth". Br J Haematol. 149 (3): 311–21. doi:10.1111/j.1365-2141.2010.08141.x. PMC 2864366. PMID 20230410.
- ↑ Kyle RA, Rajkumar SV (2004). "Multiple myeloma". N Engl J Med. 351 (18): 1860–73. doi:10.1056/NEJMra041875. PMID 15509819.
- ↑ Koura DT, Langston AA (2013). "Inherited predisposition to multiple myeloma". Ther Adv Hematol. 4 (4): 291–7. doi:10.1177/2040620713485375. PMC 3734900. PMID 23926460.
- ↑ http://picasaweb.google.com/mcmumbi/USMLEIIImages
![Multiple Myeloma slide showing plasma cells with large nucleus and scant cytoplasm [10]](/images/a/a5/Multiple_Myeloma.jpg)
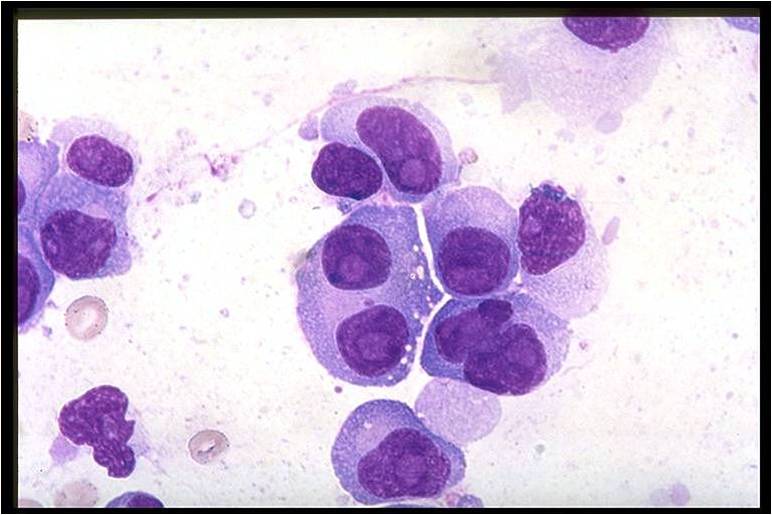
![Multiple myeloma slide with intermediate magnification[5]](/images/4/40/Multiple_myeloma_intermed_mag.jpg)
![Multiple myeloma slide with high magnification[5]](/images/d/d5/Multiple_myeloma.jpg)
![Multiple myeloma slide with russell bodies[5]](/images/a/ad/Russell_bodies.jpg)