Empty sella syndrome: Difference between revisions
Iqra Qamar (talk | contribs) |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 15: | Line 15: | ||
{{SI}} | {{SI}} | ||
'''For patient information click [[{{PAGENAME}} (patient information)|here]]'' | '''For patient information click [[{{PAGENAME}} (patient information)|here]]'' | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | ==Overview== | ||
'''Empty sella syndrome | '''Empty sella syndrome (ESS)''' is defined as herniation of [[subarachnoid space]] into the [[sella turcica]] (arachnoidocele) in which there is a characteristic radiological finding of "empty sellar space" on [[magnetic resonance imaging (MRI)]] and computerized tomography (CT) with a flattened pituitary and elongated stalk.<ref>{{Cite web|url=https://www.ncbi.nlm.nih.gov/pubmed/15972577|title=Primary empty sella.|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref> It can be partial if less than 50% of sellar space is filled with [[Cerebrospinal fluid (CSF)|cerebro-spinal fluid (CSF)]], or complete if CSF fills more than 50% of space in the sella and gland thickness is less than 2mm.<ref>{{Cite web|url=https://www.ncbi.nlm.nih.gov/pubmed/28780516|title=DIAGNOSIS OF ENDOCRINE DISEASE: Primary empty sella: a comprehensive review.|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref> | ||
==Historical Perspective== | |||
*The term ‘empty sella’ was first used by Bush in 1951<ref>{{Cite web|url=https://www.ncbi.nlm.nih.gov/pubmed/14942993|title=[Morphology of sella turcica and its relation to the pituitary gland]|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref> to describe a peculiar anatomical condition, observed in 40 of 788 human cadavers, particularly females, characterized by a sella turcica with an incomplete diaphragm sellae that forms only a small peripheral rim, with a pituitary gland not absent, but flattened in such a manner as to form a thin layer of tissue at the bottom of the sella turcica. | |||
*In [year], [gene] mutations were first identified in the pathogenesis of [disease name]. | |||
*In [year], the first [discovery] was developed by [scientist] to treat/diagnose [disease name]. | |||
==Classification== | ==Classification== | ||
*[Disease name] may be classified according to [classification method] into [number] subtypes/groups: | |||
:*[group1] | |||
:*[group2] | |||
:*[group3] | |||
*Other variants of [disease name] include [disease subtype 1], [disease subtype 2], and [disease subtype 3]. | |||
There are two types of ESS: ''primary'' and ''secondary''. | There are two types of ESS: ''primary'' and ''secondary''. | ||
* Primary ESS happens when a small anatomical defect above the pituitary gland increases pressure in the sella turica and causes the gland to flatten out along the interior walls of the sella turica cavity. Primary ESS is associated with [[obesity]] and [[high blood pressure]] in women. The disorder sometimes results in a build-up of fluid pressure inside the [[skull]] and the pituitary gland may be smaller than usual. | * Primary ESS happens when a small anatomical defect above the pituitary gland increases pressure in the sella turica and causes the gland to flatten out along the interior walls of the sella turica cavity. Primary ESS is associated with [[obesity]] and [[high blood pressure]] in women. The disorder sometimes results in a build-up of fluid pressure inside the [[skull]] and the pituitary gland may be smaller than usual. | ||
| Line 30: | Line 42: | ||
* Secondary ESS is the result of the pituitary gland regressing within the cavity after an [[injury]], [[surgery]], or [[radiation therapy]]. Individuals with secondary ESS due to destruction of the pituitary gland have symptoms that reflect the loss of pituitary functions, such as the ceasing of [[menstrual period]]s, [[infertility]], [[fatigue (physical)|fatigue]], and intolerance to stress and infection. | * Secondary ESS is the result of the pituitary gland regressing within the cavity after an [[injury]], [[surgery]], or [[radiation therapy]]. Individuals with secondary ESS due to destruction of the pituitary gland have symptoms that reflect the loss of pituitary functions, such as the ceasing of [[menstrual period]]s, [[infertility]], [[fatigue (physical)|fatigue]], and intolerance to stress and infection. | ||
== | ==Pathophysiology== | ||
*The pathogenesis of [disease name] is characterized by [feature1], [feature2], and [feature3]. | |||
*The [gene name] gene/Mutation in [gene name] has been associated with the development of [disease name], involving the [molecular pathway] pathway. | |||
*On gross pathology, [feature1], [feature2], and [feature3] are characteristic findings of [disease name]. | |||
*On microscopic histopathological analysis, [feature1], [feature2], and [feature3] are characteristic findings of [disease name]. | |||
In children, ESS may be associated with early onset of puberty, growth hormone deficiency, pituitary tumors, or pituitary gland dysfunction. MRI scans are useful in evaluating ESS and differentiating it from other disorders that produce an enlarged sella. | In children, ESS may be associated with early onset of puberty, growth hormone deficiency, pituitary tumors, or pituitary gland dysfunction. MRI scans are useful in evaluating ESS and differentiating it from other disorders that produce an enlarged sella. | ||
== | ==Clinical Features== | ||
==Differentiating [disease name] from other Diseases== | |||
*[Disease name] must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as: | |||
:*[Differential dx1] | |||
:*[Differential dx2] | |||
:*[Differential dx3] | |||
* Autoimmune factors | * Autoimmune factors | ||
* [[Cavernous sinus thrombosis]] | * [[Cavernous sinus thrombosis]] | ||
| Line 373: | Line 398: | ||
* [[Endometrial biopsy]] | * [[Endometrial biopsy]] | ||
|} | |} | ||
<small> | </small> | ||
==Epidemiology and Demographics== | |||
* The prevalence of [disease name] is approximately [number or range] per 100,000 individuals worldwide. | |||
* In [year], the incidence of [disease name] was estimated to be [number or range] cases per 100,000 individuals in [location]. | |||
===Age=== | |||
*Patients of all age groups may develop [disease name]. | |||
*[Disease name] is more commonly observed among patients aged [age range] years old. | |||
*[Disease name] is more commonly observed among [elderly patients/young patients/children]. | |||
===Gender=== | |||
*[Disease name] affects men and women equally. | |||
*[Gender 1] are more commonly affected with [disease name] than [gender 2]. | |||
* The [gender 1] to [Gender 2] ratio is approximately [number > 1] to 1. | |||
===Race=== | |||
*There is no racial predilection for [disease name]. | |||
*[Disease name] usually affects individuals of the [race 1] race. | |||
*[Race 2] individuals are less likely to develop [disease name]. | |||
==Risk Factors== | |||
*Common risk factors in the development of [disease name] are [risk factor 1], [risk factor 2], [risk factor 3], and [risk factor 4]. | |||
== Natural History, Complications and Prognosis== | |||
*The majority of patients with [disease name] remain asymptomatic for [duration/years]. | |||
*Early clinical features include [manifestation 1], [manifestation 2], and [manifestation 3]. | |||
*If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3]. | |||
*Common complications of [disease name] include [complication 1], [complication 2], and [complication 3]. | |||
*Prognosis is generally [excellent/good/poor], and the [1/5/10year mortality/survival rate] of patients with [disease name] is approximately [#%]. | |||
==Diagnosis== | == Diagnosis == | ||
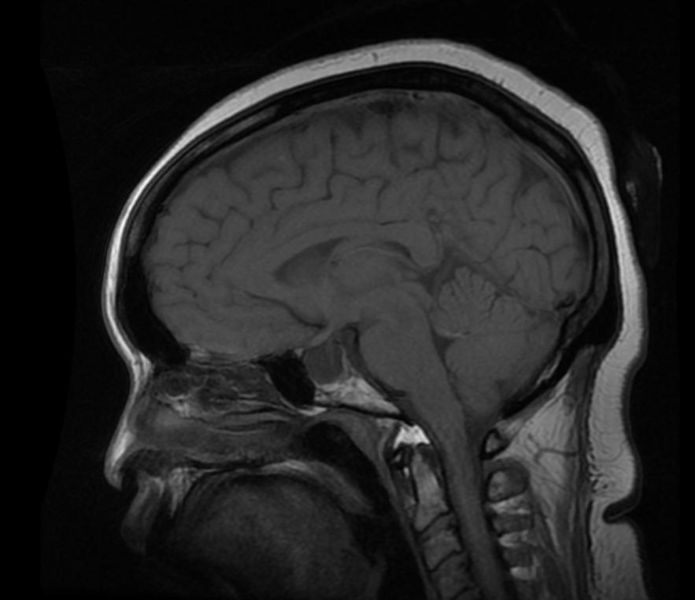
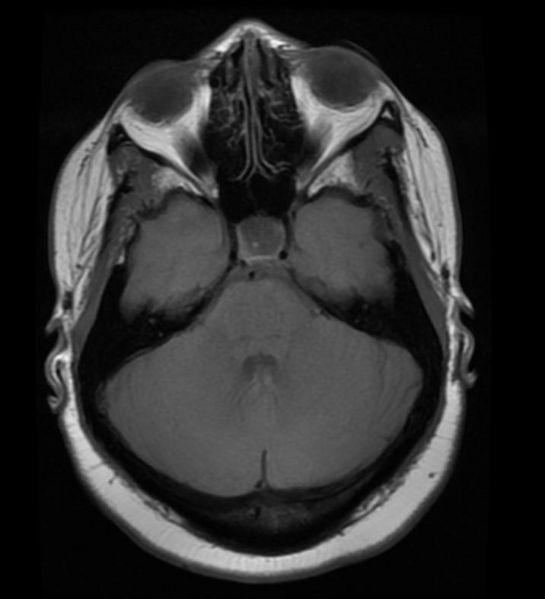
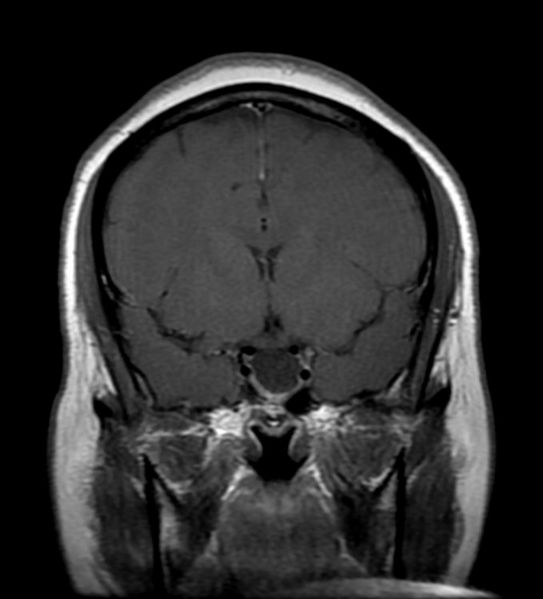
'''Patient #1: MR images demonstrate an expanded and empty sella''' | '''Patient #1: MR images demonstrate an expanded and empty sella''' | ||
<gallery> | <gallery> | ||
| Line 391: | Line 449: | ||
</gallery> | </gallery> | ||
===Diagnostic Criteria=== | |||
*The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met: | |||
:*[criterion 1] | |||
:*[criterion 2] | |||
:*[criterion 3] | |||
:*[criterion 4] | |||
=== Symptoms === | |||
*[Disease name] is usually asymptomatic. | |||
*Symptoms of [disease name] may include the following: | |||
:*[symptom 1] | |||
:*[symptom 2] | |||
:*[symptom 3] | |||
:*[symptom 4] | |||
:*[symptom 5] | |||
:*[symptom 6] | |||
=== Physical Examination === | |||
*Patients with [disease name] usually appear [general appearance]. | |||
*Physical examination may be remarkable for: | |||
:*[finding 1] | |||
:*[finding 2] | |||
:*[finding 3] | |||
:*[finding 4] | |||
:*[finding 5] | |||
:*[finding 6] | |||
=== Laboratory Findings === | |||
*There are no specific laboratory findings associated with [disease name]. | |||
*A [positive/negative] [test name] is diagnostic of [disease name]. | |||
*An [elevated/reduced] concentration of [serum/blood/urinary/CSF/other] [lab test] is diagnostic of [disease name]. | |||
*Other laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3]. | |||
===Imaging Findings=== | |||
*There are no [imaging study] findings associated with [disease name]. | |||
*[Imaging study 1] is the imaging modality of choice for [disease name]. | |||
*On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3]. | |||
*[Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3]. | |||
=== Other Diagnostic Studies === | |||
*[Disease name] may also be diagnosed using [diagnostic study name]. | |||
*Findings on [diagnostic study name] include [finding 1], [finding 2], and [finding 3]. | |||
== Treatment == | == Treatment == | ||
=== Medical Therapy === | |||
*There is no treatment for [disease name]; the mainstay of therapy is supportive care. | |||
*The mainstay of therapy for [disease name] is [medical therapy 1] and [medical therapy 2]. | |||
*[Medical therapy 1] acts by [mechanism of action 1]. | |||
*Response to [medical therapy 1] can be monitored with [test/physical finding/imaging] every [frequency/duration]. | |||
=== Surgery === | |||
*Surgery is the mainstay of therapy for [disease name]. | |||
*[Surgical procedure] in conjunction with [chemotherapy/radiation] is the most common approach to the treatment of [disease name]. | |||
*[Surgical procedure] can only be performed for patients with [disease stage] [disease name]. | |||
=== Prevention === | |||
*There are no primary preventive measures available for [disease name]. | |||
*Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3]. | |||
*Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3]. | |||
==References== | |||
{{Reflist|2}} | |||
[[Category:Pick One of 28 Approved]] | |||
{{WS}} | |||
{{WH}} | |||
== External links == | == External links == | ||
Latest revision as of 05:29, 27 March 2019
| Empty sella syndrome | |
| ICD-9 | 253.8 |
|---|---|
| DiseasesDB | 31523 |
| MeSH | D004652 |
'For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Empty sella syndrome (ESS) is defined as herniation of subarachnoid space into the sella turcica (arachnoidocele) in which there is a characteristic radiological finding of "empty sellar space" on magnetic resonance imaging (MRI) and computerized tomography (CT) with a flattened pituitary and elongated stalk.[1] It can be partial if less than 50% of sellar space is filled with cerebro-spinal fluid (CSF), or complete if CSF fills more than 50% of space in the sella and gland thickness is less than 2mm.[2]
Historical Perspective
- The term ‘empty sella’ was first used by Bush in 1951[3] to describe a peculiar anatomical condition, observed in 40 of 788 human cadavers, particularly females, characterized by a sella turcica with an incomplete diaphragm sellae that forms only a small peripheral rim, with a pituitary gland not absent, but flattened in such a manner as to form a thin layer of tissue at the bottom of the sella turcica.
- In [year], [gene] mutations were first identified in the pathogenesis of [disease name].
- In [year], the first [discovery] was developed by [scientist] to treat/diagnose [disease name].
Classification
- [Disease name] may be classified according to [classification method] into [number] subtypes/groups:
- [group1]
- [group2]
- [group3]
- Other variants of [disease name] include [disease subtype 1], [disease subtype 2], and [disease subtype 3].
There are two types of ESS: primary and secondary.
- Primary ESS happens when a small anatomical defect above the pituitary gland increases pressure in the sella turica and causes the gland to flatten out along the interior walls of the sella turica cavity. Primary ESS is associated with obesity and high blood pressure in women. The disorder sometimes results in a build-up of fluid pressure inside the skull and the pituitary gland may be smaller than usual.
- Secondary ESS is the result of the pituitary gland regressing within the cavity after an injury, surgery, or radiation therapy. Individuals with secondary ESS due to destruction of the pituitary gland have symptoms that reflect the loss of pituitary functions, such as the ceasing of menstrual periods, infertility, fatigue, and intolerance to stress and infection.
Pathophysiology
- The pathogenesis of [disease name] is characterized by [feature1], [feature2], and [feature3].
- The [gene name] gene/Mutation in [gene name] has been associated with the development of [disease name], involving the [molecular pathway] pathway.
- On gross pathology, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
- On microscopic histopathological analysis, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
In children, ESS may be associated with early onset of puberty, growth hormone deficiency, pituitary tumors, or pituitary gland dysfunction. MRI scans are useful in evaluating ESS and differentiating it from other disorders that produce an enlarged sella.
Clinical Features
Differentiating [disease name] from other Diseases
- [Disease name] must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as:
- [Differential dx1]
- [Differential dx2]
- [Differential dx3]
- Autoimmune factors
- Cavernous sinus thrombosis
- Communicating hydrocephalus
- Congenital absence of the diaphragma sellae
- Diabetes Mellitus
- Familial
- Head trauma
- Increased intracranial pressure
- Radiation therapy complication
- Meningitis
- Pituitary adenoma
- Primary in middle-aged obese women
- Rupture of intrasellar or parasellar cyst
- Sheehan's Syndrome
- Surgery
- Vascular diseases
Differentiating Empty sella syndrome from other diseases:
Empty sella syndrome needs to be differentiated from other diseases causing hypopituitarism.[4][5][6][7][8][9][10]
| Diseases | Onset | Manifestations | Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| History and Symptoms | Physical examination | Laboratory findings | Gold standard | Imaging | Other investigation findings | |||||
| Trumatic delivery | Lactation failure | Menstrual irregularities | Other features | |||||||
| Sheehan's syndrome | Acute | ++ | ++ | Oligo/amenorrhea | Symptoms of: |
|
|
CT/MRI:
|
| |
| Lymphocytic hypophysitis | Acute | +/- | + | Oligo/amenorrhea |
|
|
|
Assays for:
| ||
| Pituitary apoplexy | Acute | +/- | ++ | Oligo/amenorrhea | Severe headache
|
|
|
Blood tests may be done to check: | ||
| Empty sella syndrome | Chronic | - | + | Oligo/amenorrhea |
|
|
|
|
| |
| Simmonds' disease/Pituitary cachexia | Chronic | +/- | + | Oligo/amenorrhea |
|
|
|
| ||
| Hypothyroidism | Chronic | +/- | - | Oligomenorrhea/menorrhagia |
|
|
|
|
|
|
| Hypogonadotropic hypogonadism | Chronic | - | - | Oligo/amenorrhea |
|
|
|
|
| |
| Hypoprolactinemia | Chronic | - | + | - |
|
|
|
|
|
|
| Panhypopituitarism | Chronic | - | + | Oligo/amenorrhea |
|
|
|
|
| |
| Primary adrenal insufficiency/Addison's disease | Chronic | - | - | - |
|
|
|
| ||
| Menopause | Chronic | - | +/- | Oligo/amenorrhea |
|
|
Normal | |||
Epidemiology and Demographics
- The prevalence of [disease name] is approximately [number or range] per 100,000 individuals worldwide.
- In [year], the incidence of [disease name] was estimated to be [number or range] cases per 100,000 individuals in [location].
Age
- Patients of all age groups may develop [disease name].
- [Disease name] is more commonly observed among patients aged [age range] years old.
- [Disease name] is more commonly observed among [elderly patients/young patients/children].
Gender
- [Disease name] affects men and women equally.
- [Gender 1] are more commonly affected with [disease name] than [gender 2].
- The [gender 1] to [Gender 2] ratio is approximately [number > 1] to 1.
Race
- There is no racial predilection for [disease name].
- [Disease name] usually affects individuals of the [race 1] race.
- [Race 2] individuals are less likely to develop [disease name].
Risk Factors
- Common risk factors in the development of [disease name] are [risk factor 1], [risk factor 2], [risk factor 3], and [risk factor 4].
Natural History, Complications and Prognosis
- The majority of patients with [disease name] remain asymptomatic for [duration/years].
- Early clinical features include [manifestation 1], [manifestation 2], and [manifestation 3].
- If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
- Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
- Prognosis is generally [excellent/good/poor], and the [1/5/10year mortality/survival rate] of patients with [disease name] is approximately [#%].
Diagnosis
Patient #1: MR images demonstrate an expanded and empty sella
Diagnostic Criteria
- The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met:
- [criterion 1]
- [criterion 2]
- [criterion 3]
- [criterion 4]
Symptoms
- [Disease name] is usually asymptomatic.
- Symptoms of [disease name] may include the following:
- [symptom 1]
- [symptom 2]
- [symptom 3]
- [symptom 4]
- [symptom 5]
- [symptom 6]
Physical Examination
- Patients with [disease name] usually appear [general appearance].
- Physical examination may be remarkable for:
- [finding 1]
- [finding 2]
- [finding 3]
- [finding 4]
- [finding 5]
- [finding 6]
Laboratory Findings
- There are no specific laboratory findings associated with [disease name].
- A [positive/negative] [test name] is diagnostic of [disease name].
- An [elevated/reduced] concentration of [serum/blood/urinary/CSF/other] [lab test] is diagnostic of [disease name].
- Other laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3].
Imaging Findings
- There are no [imaging study] findings associated with [disease name].
- [Imaging study 1] is the imaging modality of choice for [disease name].
- On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3].
- [Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3].
Other Diagnostic Studies
- [Disease name] may also be diagnosed using [diagnostic study name].
- Findings on [diagnostic study name] include [finding 1], [finding 2], and [finding 3].
Treatment
Medical Therapy
- There is no treatment for [disease name]; the mainstay of therapy is supportive care.
- The mainstay of therapy for [disease name] is [medical therapy 1] and [medical therapy 2].
- [Medical therapy 1] acts by [mechanism of action 1].
- Response to [medical therapy 1] can be monitored with [test/physical finding/imaging] every [frequency/duration].
Surgery
- Surgery is the mainstay of therapy for [disease name].
- [Surgical procedure] in conjunction with [chemotherapy/radiation] is the most common approach to the treatment of [disease name].
- [Surgical procedure] can only be performed for patients with [disease stage] [disease name].
Prevention
- There are no primary preventive measures available for [disease name].
- Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3].
- Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3].
References
- ↑ "Primary empty sella".
- ↑ "DIAGNOSIS OF ENDOCRINE DISEASE: Primary empty sella: a comprehensive review".
- ↑ "[Morphology of sella turcica and its relation to the pituitary gland]".
- ↑ Sato N, Sze G, Endo K (1998). "Hypophysitis: endocrinologic and dynamic MR findings". AJNR Am J Neuroradiol. 19 (3): 439–44. PMID 9541295.
- ↑ Powrie JK, Powell M, Ayers AB, Lowy C, Sönksen PH (1995). "Lymphocytic adenohypophysitis: magnetic resonance imaging features of two new cases and a review of the literature". Clin. Endocrinol. (Oxf). 42 (3): 315–22. PMID 7758238.
- ↑ Honegger J, Schlaffer S, Menzel C, Droste M, Werner S, Elbelt U, Strasburger C, Störmann S, Küppers A, Streetz-van der Werf C, Deutschbein T, Stieg M, Rotermund R, Milian M, Petersenn S (2015). "Diagnosis of Primary Hypophysitis in Germany". J. Clin. Endocrinol. Metab. 100 (10): 3841–9. doi:10.1210/jc.2015-2152. PMID 26262437.
- ↑ Thodou E, Asa SL, Kontogeorgos G, Kovacs K, Horvath E, Ezzat S (1995). "Clinical case seminar: lymphocytic hypophysitis: clinicopathological findings". J. Clin. Endocrinol. Metab. 80 (8): 2302–11. doi:10.1210/jcem.80.8.7629223. PMID 7629223.
- ↑ Imura H, Nakao K, Shimatsu A, Ogawa Y, Sando T, Fujisawa I, Yamabe H (1993). "Lymphocytic infundibuloneurohypophysitis as a cause of central diabetes insipidus". N. Engl. J. Med. 329 (10): 683–9. doi:10.1056/NEJM199309023291002. PMID 8345854.
- ↑ Hsieh CY, Liu BY, Yang YN, Yin WH, Young MS (2011). "Massive pericardial effusion with diastolic right ventricular compression secondary to hypothyroidism in a 73-year-old woman". Emerg Med Australas. 23 (3): 372–5. doi:10.1111/j.1742-6723.2011.01425.x. PMID 21668725.
- ↑ Dejager S, Gerber S, Foubert L, Turpin G (1998). "Sheehan's syndrome: differential diagnosis in the acute phase". J. Intern. Med. 244 (3): 261–6. PMID 9747750.