Efavirenz
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Efavirenz is a non-nucleoside reverse transcriptase inhibitor that is FDA approved for the treatment of HIV-1 in adults and children at least 3 months old and weighing at least 3.5 kg. Common adverse reactions include impaired concentration, abnormal dreams, rash, dizziness, nausea, headache, fatigue, insomnia and vomiting..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
HIV Infection
The recommended dosage of Efavirenz is 600 mg orally, once daily, in combination with a protease inhibitor and/or nucleoside analogue reverse transcriptase inhibitors (NRTIs). It is recommended that SUSTIVA be taken on an empty stomach, preferably at bedtime. The increased efavirenz concentrations observed following administration of SUSTIVA with food may lead to an increase in frequency of adverse reactions. Dosing at bedtime may improve the tolerability of nervous system symptoms. SUSTIVA capsules or tablets should be swallowed intact with liquid. For patients who cannot swallow capsules or tablets, the capsule sprinkle method of administration is recommended.
Concomitant Antiretroviral Therapy
- SUSTIVA must be given in combination with other antiretroviral medications
DosageAdjustment
- If SUSTIVA is coadministered with voriconazole, the voriconazole maintenance dose should be increased to 400 mg every 12 hours and the SUSTIVA dose should be decreased to 300 mg once daily using the capsule formulation (one 200 mg and two 50 mg capsules or six 50 mg capsules). SUSTIVA tablets should not be broken.
- If SUSTIVA is coadministered with rifampin to patients weighing 50 kg or more, an increase in the dose of SUSTIVA to 800 mg once daily is recommended
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Efavirenz in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Efavirenz in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
HIV Infection
It is recommended that SUSTIVA be taken on an empty stomach, preferably at bedtime. Table 1 describes the recommended dose of SUSTIVA for pediatric patients 3 months of age or older and weighing between 3.5 kg and 40 kg. The recommended dosage of SUSTIVA for pediatric patients weighing 40 kg or greater is 600 mg once daily. For pediatric patients who cannot swallow capsules, the capsule contents can be administered with a small amount of food or infant formula using the capsule sprinkle method of administration.

Capsule Sprinkle Method of Administration
For pediatric patients at least 3 months old and weighing at least 3.5 kg and adults who cannot swallow capsules or tablets, the capsule contents may be administered with a small amount (1 to 2 teaspoons) of food. Use of infant formula for mixing should only be considered for those young infants who cannot reliably consume solid foods. Patients and caregivers must be instructed to open the capsule carefully to avoid spillage or dispersion of the capsule contents into the air. The capsule should be held horizontally over a small container and carefully twisted to open. For patients able to tolerate solid foods, the entire capsule contents should be gently mixed with an age-appropriate soft food, such as applesauce, grape jelly, or yogurt, in the small container. For young infants receiving the capsule sprinkle-infant formula mixture, the entire capsule contents should be gently mixed into 2 teaspoons (10 mL) of reconstituted room temperature infant formula in a medicine cup by carefully stirring with a small spoon, and then drawing up the mixture into a 10 mL oral dosing syringe for administration. After administration of the SUSTIVA-food or -formula mixture, an additional small amount (approximately 2 teaspoons) of food or formula must be added to the empty mixing container, stirred to disperse any remaining SUSTIVA residue, and administered to the patient. The SUSTIVA-food or -formula mixture should be administered within 30 minutes of mixing. No additional food should be consumed for 2 hours after administration of SUSTIVA.
Further patient instructions on the capsule sprinkle method of administration are provided in the FDA-approved patient labeling (see Patient Information and Instructions for Use).
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Efavirenz in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Efavirenz in pediatric patients.
Contraindications
Hypersensitivity
SUSTIVA is contraindicated in patients with previously demonstrated clinically significant hypersensitivity (eg, Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to any of the components of this product.
Warnings
Drug Interactions
Efavirenz plasma concentrations may be altered by substrates, inhibitors, or inducers of CYP3A. Likewise, efavirenz may alter plasma concentrations of drugs metabolized by CYP3A or CYP2B6. The most prominent effect of efavirenz at steady-state is induction of CYP3A and CYP2B6.
Resistance
SUSTIVA must not be used as a single agent to treat HIV-1 infection or added on as a sole agent to a failing regimen. Resistant virus emerges rapidly when efavirenz is administered as monotherapy. The choice of new antiretroviral agents to be used in combination with efavirenz should take into consideration the potential for viral cross-resistance.
Coadministration with Related Products
Coadministration of SUSTIVA with ATRIPLA (efavirenz 600 mg/emtricitabine 200 mg/tenofovir disoproxil fumarate 300 mg) is not recommended unless needed for dose adjustment (eg, with rifampin), since efavirenz is one of its active ingredients.
Psychiatric Symptoms
Serious psychiatric adverse experiences have been reported in patients treated with SUSTIVA. In controlled trials of 1008 patients treated with regimens containing SUSTIVA for a mean of 2.1 years and 635 patients treated with control regimens for a mean of 1.5 years, the frequency (regardless of causality) of specific serious psychiatric events among patients who received SUSTIVA or control regimens, respectively, were severe depression (2.4%, 0.9%), suicidal ideation (0.7%, 0.3%), nonfatal suicide attempts (0.5%, 0), aggressive behavior (0.4%, 0.5%), paranoid reactions (0.4%, 0.3%), and manic reactions (0.2%, 0.3%). When psychiatric symptoms similar to those noted above were combined and evaluated as a group in a multifactorial analysis of data from Study 006, treatment with efavirenz was associated with an increase in the occurrence of these selected psychiatric symptoms. Other factors associated with an increase in the occurrence of these psychiatric symptoms were history of injection drug use, psychiatric history, and receipt of psychiatric medication at study entry; similar associations were observed in both the SUSTIVA and control treatment groups. In Study 006, onset of new serious psychiatric symptoms occurred throughout the study for both SUSTIVA-treated and control-treated patients. One percent of SUSTIVA-treated patients discontinued or interrupted treatment because of one or more of these selected psychiatric symptoms. There have also been occasional postmarketing reports of death by suicide, delusions, and psychosis-like behavior, although a causal relationship to the use of SUSTIVA cannot be determined from these reports. Patients with serious psychiatric adverse experiences should seek immediate medical evaluation to assess the possibility that the symptoms may be related to the use of SUSTIVA, and if so, to determine whether the risks of continued therapy outweigh the benefits.
Nervous System Symptoms
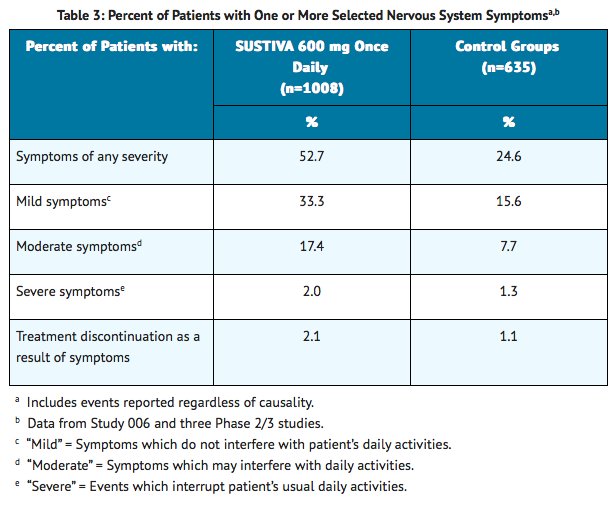
Fifty-three percent (531/1008) of patients receiving SUSTIVA in controlled trials reported central nervous system symptoms (any grade, regardless of causality) compared to 25% (156/635) of patients receiving control regimens. These symptoms included, but were not limited to, dizziness (28.1% of the 1008 patients), insomnia (16.3%), impaired concentration (8.3%), somnolence (7.0%), abnormal dreams (6.2%), and hallucinations (1.2%). These symptoms were severe in 2.0% of patients, and 2.1% of patients discontinued therapy as a result. These symptoms usually begin during the first or second day of therapy and generally resolve after the first 2-4 weeks of therapy. After 4 weeks of therapy, the prevalence of nervous system symptoms of at least moderate severity ranged from 5% to 9% in patients treated with regimens containing SUSTIVA and from 3% to 5% in patients treated with a control regimen. Patients should be informed that these common symptoms were likely to improve with continued therapy and were not predictive of subsequent onset of the less frequent psychiatric symptoms. Dosing at bedtime may improve the tolerability of these nervous system symptoms.
- Analysis of long-term data from Study 006 (median follow-up 180 weeks, 102 weeks, and 76 weeks for patients treated with SUSTIVA + zidovudine + lamivudine, SUSTIVA + indinavir, and indinavir + zidovudine + lamivudine, respectively) showed that, beyond 24 weeks of therapy, the incidences of new-onset nervous system symptoms among SUSTIVA-treated patients were generally similar to those in the indinavir-containing control arm.
- Patients receiving SUSTIVA should be alerted to the potential for additive central nervous system effects when SUSTIVA is used concomitantly with alcohol or psychoactive drugs.
- Patients who experience central nervous system symptoms such as dizziness, impaired concentration, and/or drowsiness should avoid potentially hazardous tasks such as driving or operating machinery.
Reproductive Risk Potential
Pregnancy Category D. Efavirenz may cause fetal harm when administered during the first trimester to a pregnant woman. Pregnancy should be avoided in women receiving SUSTIVA. Barrier contraception must always be used in combination with other methods of contraception (eg, oral or other hormonal contraceptives). Because of the long half-life of efavirenz, use of adequate contraceptive measures for 12 weeks after discontinuation of SUSTIVA is recommended. Women of childbearing potential should undergo pregnancy testing before initiation of SUSTIVA. If this drug is used during the first trimester of pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential harm to the fetus. There are no adequate and well-controlled studies in pregnant women. SUSTIVA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus, such as in pregnant women without other therapeutic options.
Rash
In controlled clinical trials, 26% (266/1008) of adult patients treated with 600 mg SUSTIVA experienced new-onset skin rash compared with 17% (111/635) of those treated in control groups. Rash associated with blistering, moist desquamation, or ulceration occurred in 0.9% (9/1008) of patients treated with SUSTIVA. The incidence of Grade 4 rash (eg, erythema multiforme, Stevens-Johnson syndrome) in adult patients treated with SUSTIVA in all studies and expanded access was 0.1%. Rashes are usually mild-to-moderate maculopapular skin eruptions that occur within the first 2 weeks of initiating therapy with efavirenz (median time to onset of rash in adults was 11 days) and, in most patients continuing therapy with efavirenz, rash resolves within 1 month (median duration, 16 days). The discontinuation rate for rash in adult clinical trials was 1.7% (17/1008).
Rash was reported in 59 of 182 pediatric patients (32%) treated with SUSTIVA. Two pediatric patients experienced Grade 3 rash (confluent rash with fever, generalized rash), and four patients had Grade 4 rash (erythema multiforme). The median time to onset of rash in pediatric patients was 28 days (range 3-1642 days). Prophylaxis with appropriate antihistamines before initiating therapy with SUSTIVA in pediatric patients should be considered.
SUSTIVA can be reinitiated in patients interrupting therapy because of rash. SUSTIVA should be discontinued in patients developing severe rash associated with blistering, desquamation, mucosal involvement, or fever. Appropriate antihistamines and/or corticosteroids may improve the tolerability and hasten the resolution of rash. For patients who have had a life-threatening cutaneous reaction (eg, Stevens-Johnson syndrome), alternative therapy should be considered.
Hepatotoxicity
Monitoring of liver enzymes before and during treatment is recommended for patients with underlying hepatic disease, including hepatitis B or hepatitis C infection; patients with marked transaminase elevations; and patients treated with other medications associated with liver toxicity. A few of the postmarketing reports of hepatic failure occurred in patients with no pre-existing hepatic disease or other identifiable risk factors. Liver enzyme monitoring should also be considered for patients without pre-existing hepatic dysfunction or other risk factors. In patients with persistent elevations of serum transaminases to greater than five times the upper limit of the normal range, the benefit of continued therapy with SUSTIVA needs to be weighed against the unknown risks of significant liver toxicity.
Convulsions
Convulsions have been observed in adult and pediatric patients receiving efavirenz, generally in the presence of known medical history of seizures. Caution must be taken in any patient with a history of seizures. Patients who are receiving concomitant anticonvulsant medications primarily metabolized by the liver, such as phenytoin and phenobarbital, may require periodic monitoring of plasma levels.
Lipid Elevations
Treatment with SUSTIVA has resulted in increases in the concentration of total cholesterol and triglycerides. Cholesterol and triglyceride testing should be performed before initiating SUSTIVA therapy and at periodic intervals during therapy.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including SUSTIVA. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Adverse Reactions
Clinical Trials Experience
Adults
Because clinical studies are conducted under widely varying conditions, the adverse reaction rates reported cannot be directly compared to rates in other clinical studies and may not reflect the rates observed in clinical practice. The most significant adverse reactions observed in patients treated with SUSTIVA are:
- Psychiatric symptoms
- Nervous system symptoms
- Rash
- The most common (>5% in either efavirenz treatment group) adverse reactions of at least moderate severity among patients in Study 006 treated with SUSTIVA in combination with zidovudine/lamivudine or indinavir were rash, dizziness, nausea, headache, fatigue, insomnia, and vomiting. Selected clinical adverse reactions of moderate or severe intensity observed in ≥2% of SUSTIVA-treated patients in two controlled clinical trials are presented in Table 2.
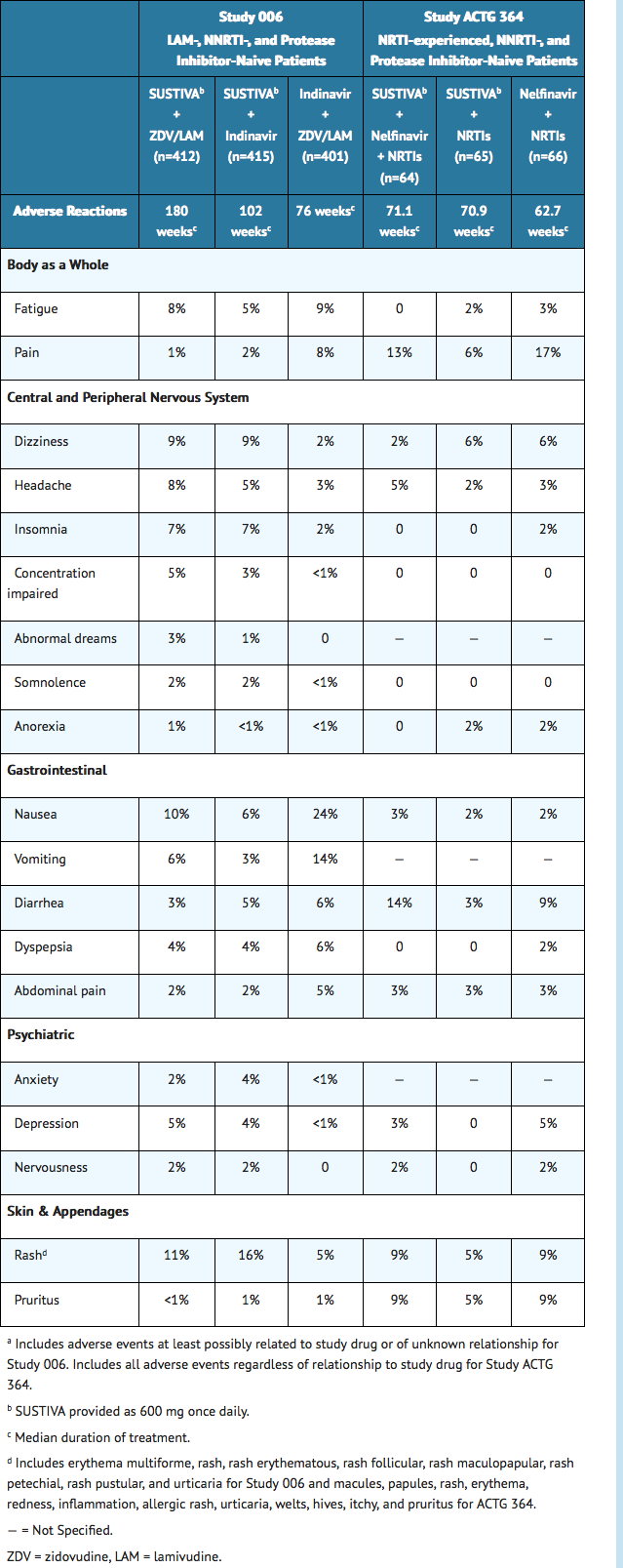
Pancreatitis has been reported, although a causal relationship with efavirenz has not been established. Asymptomatic increases in serum amylase levels were observed in a significantly higher number of patients treated with efavirenz 600 mg than in control patients.
Central Nervous System
For 1008 patients treated with regimens containing SUSTIVA and 635 patients treated with a control regimen in controlled trials, Table 3 lists the frequency of symptoms of different degrees of severity and gives the discontinuation rates for one or more of the following nervous system symptoms: dizziness, insomnia, impaired concentration, somnolence, abnormal dreaming, euphoria, confusion, agitation, amnesia, hallucinations, stupor, abnormal thinking and depersonalization. The frequencies of specific central and peripheral nervous system symptoms are provided in Table 2.
Psychiatric Symptoms
Serious psychiatric adverse experiences have been reported in patients treated with SUSTIVA. In controlled trials, psychiatric symptoms observed at a frequency greater than 2% among patients treated with SUSTIVA or control regimens, respectively, were depression (19%, 16%), anxiety (13%, 9%), and nervousness (7%, 2%).
Rash
In controlled clinical trials, the frequency of rash (all grades, regardless of causality) was 26% for 1008 adults treated with regimens containing SUSTIVA and 17% for 635 adults treated with a control regimen. Most reports of rash were mild or moderate in severity. The frequency of Grade 3 rash was 0.8% for SUSTIVA-treated patients and 0.3% for control groups, and the frequency of Grade 4 rash was 0.1% for SUSTIVA and 0 for control groups. The discontinuation rates as a result of rash were 1.7% for SUSTIVA-treated patients and 0.3% for control groups.
Experience with SUSTIVA in patients who discontinued other antiretroviral agents of the NNRTI class is limited. Nineteen patients who discontinued nevirapine because of rash have been treated with SUSTIVA. Nine of these patients developed mild-to-moderate rash while receiving therapy with SUSTIVA, and two of these patients discontinued because of rash.
Laboratory Abnormalities
Selected Grade 3-4 laboratory abnormalities reported in ≥2% of SUSTIVA-treated patients in two clinical trials are presented in Table 4.
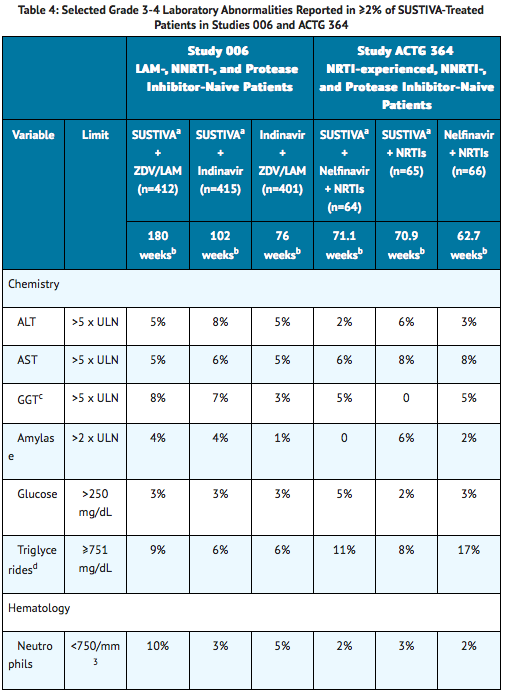
Patients Coinfected with Hepatitis B or C
Liver function tests should be monitored in patients with a history of hepatitis B and/or hepatitis C. In the long-term data set from Study 006, 137 patients treated with SUSTIVA-containing regimens (median duration of therapy, 68 weeks) and 84 treated with a control regimen (median duration, 56 weeks) were seropositive at screening for hepatitis B (surface antigen positive) and/or hepatitis C (hepatitis C antibody positive). Among these coinfected patients, elevations in AST to greater than five times ULN developed in 13% of patients in the SUSTIVA arms and 7% of those in the control arm, and elevations in ALT to greater than five times ULN developed in 20% of patients in the SUSTIVA arms and 7% of patients in the control arm. Among coinfected patients, 3% of those treated with SUSTIVA-containing regimens and 2% in the control arm discontinued from the study because of liver or biliary system disorders.
Lipids
Increases from baseline in total cholesterol of 10-20% have been observed in some uninfected volunteers receiving SUSTIVA. In patients treated with SUSTIVA + zidovudine + lamivudine, increases from baseline in nonfasting total cholesterol and HDL of approximately 20% and 25%, respectively, were observed. In patients treated with SUSTIVA + indinavir, increases from baseline in non fasting cholesterol and HDL of approximately 40% and 35%, respectively, were observed. Nonfasting total cholesterol levels ≥240 mg/dL and ≥300 mg/dL were reported in 34% and 9%, respectively, of patients treated with SUSTIVA + zidovudine + lamivudine; 54% and 20%, respectively, of patients treated with SUSTIVA + indinavir; and 28% and 4%, respectively, of patients treated with indinavir + zidovudine + lamivudine. The effects of SUSTIVA on triglycerides and LDL in this study were not well characterized since samples were taken from nonfasting patients. The clinical significance of these findings is unknown.
Clinical Trial Experience in Pediatric Patients
Because clinical studies are conducted under widely varying conditions, the adverse reaction rates reported cannot be directly compared to rates in other clinical studies and may not reflect the rates observed in clinical practice. Assessment of adverse reactions is based on three clinical trials in 182 HIV-1 infected pediatric patients (3 months to 21 years of age) who received SUSTIVA in combination with other antiretroviral agents for a median of 123 weeks. The adverse reactions observed in the three trials were similar to those observed in clinical trials in adults except that rash was more common in pediatric patients (32% for all grades regardless of causality) and more often of higher grade (ie, more severe). Two (1.1%) pediatric patients experienced Grade 3 rash (confluent rash with fever, generalized rash), and four (2.2%) pediatric patients had Grade 4 rash (all erythema multiforme). Five pediatric patients (2.7%) discontinued from the study because of rash.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of SUSTIVA. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole
- Allergic reactions
- Asthenia
- Redistribution/accumulation of body fat
Central and Peripheral Nervous System
- Abnormal coordination
- Ataxia
- Cerebellar coordination and balance disturbances
- Convulsions
- Hypoesthesia
- Paresthesia
- Neuropathy
- Tremor
- Vertigo
Endocrine
Gastrointestinal
Cardiovascular
Liver and Biliary System
- Hepatic enzyme increase
- Hepatic failure
- Hepatitis
- A few of the postmarketing reports of hepatic failure, including cases in patients with no pre-existing hepatic disease or other identifiable risk factors, were characterized by a fulminant course, progressing in some cases to transplantation or death.
Metabolic and Nutritional
Musculoskeletal
Psychiatric
- Aggressive reactions
- Agitation
- Delusions
- Emotional lability
- Mania
- Neurosis
- Paranoia
- Psychosis
- Suicide
Respiratory
Skin and Appendages
Special Senses
Drug Interactions
Drug-Drug Interactions
Efavirenz has been shown in vivo to induce CYP3A and CYP2B6. Other compounds that are substrates of CYP3A or CYP2B6 may have decreased plasma concentrations when coadministered with SUSTIVA. Drugs that induce CYP3A activity (eg, phenobarbital, rifampin, rifabutin) would be expected to increase the clearance of efavirenz resulting in lowered plasma concentrations. Drug interactions with SUSTIVA are summarized in Table 5. This table includes potentially significant interactions, but is not all inclusive.
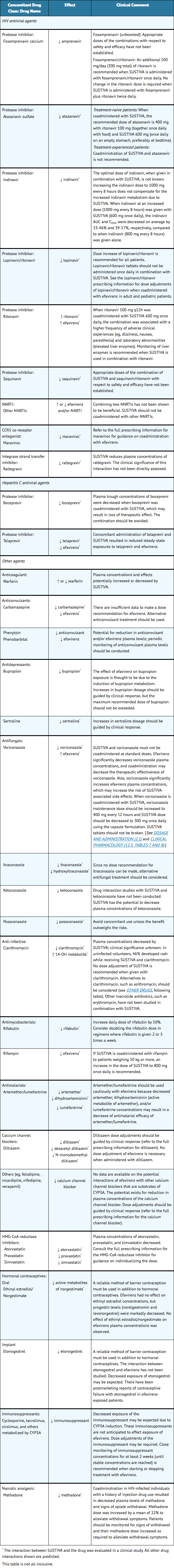
Other Drugs
Based on the results of drug interaction studies, no dosage adjustment is recommended when SUSTIVA is given with the following: aluminum/][magnesium hydroxide]] antacids, azithromycin, cetirizine, famotidine, fluconazole, lamivudine, lorazepam, nelfinavir, paroxetine, tenofovir, disoproxil fumarate, and zidovudine.
Specific drug interaction studies have not been performed with SUSTIVA and NRTIs other than lamivudine and zidovudine. Clinically significant interactions would not be expected since the NRTIs are metabolized via a different route than efavirenz and would be unlikely to compete for the same metabolic enzymes and elimination pathways.
Cannabinoid Test Interaction
Efavirenz does not bind to cannabinoid receptors. False-positive urine cannabinoid test results have been reported with some screening assays in uninfected and HIV-infected subjects receiving efavirenz. Confirmation of positive screening tests for cannabinoids by a more specific method is recommended.
Use in Specific Populations
Pregnancy
Antiretroviral Pregnancy Registry
To monitor fetal outcomes of pregnant women exposed to SUSTIVA, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
As of July 2010, the Antiretroviral Pregnancy Registry has received prospective reports of 792 pregnancies exposed to efavirenz-containing regimens, nearly all of which were first-trimester exposures (718 pregnancies). Birth defects occurred in 17 of 604 live births (first-trimester exposure) and 2 of 69 live births (second/third-trimester exposure). One of these prospectively reported defects with first-trimester exposure was a neural tube defect. A single case of anophthalmia with first-trimester exposure to efavirenz has also been prospectively reported; however, this case included severe oblique facial clefts and amniotic banding, a known association with anophthalmia. There have been six retrospective reports of findings consistent with neural tube defects, including meningomyelocele. All mothers were exposed to efavirenz-containing regimens in the first trimester. Although a causal relationship of these events to the use of SUSTIVA has not been established, similar defects have been observed in preclinical studies of efavirenz.
Animal Data
Effects of efavirenz on embryo-fetal development have been studied in three nonclinical species (cynomolgus monkeys, rats, and rabbits). In monkeys, efavirenz 60 mg/kg/day was administered to pregnant females throughout pregnancy (gestation days 20 through 150). The maternal systemic drug exposures (AUC) were 1.3 times the exposure in humans at the recommended clinical dose (600 mg/day), with fetal umbilical venous drug concentrations approximately 0.7 times the maternal values. Three fetuses of 20 fetuses/infants had one or more malformations; there were no malformed fetuses or infants from placebo-treated mothers. The malformations that occurred in these three monkey fetuses included anencephaly and unilateral anophthalmia in one fetus, microphthalmia in a second, and cleft palate in the third. There was no NOAEL (no observable adverse effect level) established for this study because only one dosage was evaluated. In rats, efavirenz was administered either during organogenesis (gestation days 7 to 18) or from gestation day 7 through lactation day 21 at 50, 100, or 200 mg/kg/day. Administration of 200 mg/kg/day in rats was associated with increase in the incidence of early resorptions; and doses 100 mg/kg/day and greater were associated with early neonatal mortality. The AUC at the NOAEL (50 mg/kg/day) in this rat study was 0.1 times that in humans at the recommended clinical dose. Drug concentrations in the milk on lactation day 10 were approximately 8 times higher than those in maternal plasma. In pregnant rabbits, efavirenz was neither embryo lethal nor teratogenic when administered at doses of 25, 50, and 75 mg/kg/day over the period of organogenesis (gestation days 6 through 18). The AUC at the NOAEL (75 mg/kg/day) in rabbits was 0.4 times that in humans at the recommended clinical dose.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Efavirenz in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Efavirenz during labor and delivery.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Efavirenz has been shown to pass into human breast milk. Because of the potential for HIV transmission and the potential for serious adverse effects in nursing infants, mothers should be instructed not to breastfeed if they are receiving SUSTIVA.
Pediatric Use
The safety, pharmacokinetic profile, and virologic and immunologic responses of SUSTIVA were evaluated in antiretroviral-naive and -experienced HIV-1 infected pediatric patients 3 months to 21 years of age in three open-label clinical trials. The type and frequency of adverse reactions in these trials were generally similar to those of adult patients with the exception of a higher frequency of rash, including a higher frequency of Grade 3 or 4 rash, in pediatric patients compared to adults.
Use of SUSTIVA in patients younger than 3 months of age OR less than 3.5 kg body weight is not recommended because the safety, pharmacokinetics, and antiviral activity of SUSTIVA have not been evaluated in this age group and there is a risk of developing HIV resistance if SUSTIVA is underdosed.
Geriatic Use
Clinical studies of SUSTIVA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other therapy.
Gender
There is no FDA guidance on the use of Efavirenz with respect to specific gender populations.
Race
There is no FDA guidance on the use of Efavirenz with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Efavirenz in patients with renal impairment.
Hepatic Impairment
SUSTIVA is not recommended for patients with moderate or severe hepatic impairment because there are insufficient data to determine whether dose adjustment is necessary. Patients with mild hepatic impairment may be treated with efavirenz without any adjustment in dose. Because of the extensive cytochrome P450-mediated metabolism of efavirenz and limited clinical experience in patients with hepatic impairment, caution should be exercised in administering SUSTIVA to these patients.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Efavirenz in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Efavirenz in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Efavirenz Administration in the drug label.
Monitoring
There is limited information regarding Efavirenz Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Efavirenz and IV administrations.
Overdosage
There is limited information regarding Efavirenz overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Efavirenz Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Efavirenz Mechanism of Action in the drug label.
Structure
There is limited information regarding Efavirenz Structure in the drug label.
Pharmacodynamics
There is limited information regarding Efavirenz Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Efavirenz Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Efavirenz Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Efavirenz Clinical Studies in the drug label.
How Supplied
There is limited information regarding Efavirenz How Supplied in the drug label.
Storage
There is limited information regarding Efavirenz Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Efavirenz |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Efavirenz |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Efavirenz Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Efavirenz interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Efavirenz Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Efavirenz Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [4]
Overview
Efavirenz (EFV, brand names Sustiva, Stocrin, Efavir etc.) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and is used as part of highly active antiretroviral therapy (HAART) for the treatment of a human immunodeficiency virus (HIV) type 1.
For HIV infection that has not previously been treated, the United States Department of Health and Human Services Panel on Antiretroviral Guidelines currently recommends the use of efavirenz in combination with tenofovir/emtricitabine (Truvada) as one of the preferred NNRTI-based regimens in adults and adolescents.[1]
Efavirenz is also used in combination with other antiretroviral agents as part of an expanded postexposure prophylaxis regimen to reduce the risk of HIV infection in people exposed to a significant risk (e.g. needlestick injuries, certain types of unprotected sex etc.).
The usual adult dose is 600 mg once a day. It is usually taken on an empty stomach at bedtime to reduce neurological and psychiatric adverse effects.
Efavirenz was combined with the popular HIV medication Truvada, which consists of tenofovir and emtricitabine, all of which are reverse transcriptase inhibitors. This combination of three medications approved by the U.S. Food and Drug Administration (FDA) in July 2006 under the brand name Atripla, provides HAART in a single tablet taken once a day. It results in a simplified drug regimen for many patients.
Category
Antiretroviral
US Brand Names
SUSTIVA®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Historical Perspective
Efavirenz was approved by the FDA on September 21, 1998, making it the 14th approved antiretroviral drug.
Recreational use
Abuse of efavirenz by crushing and smoking the tablets for supposed hallucinogenic and dissociative effects has been reported in South Africa, where it is used in a mixture known as whoonga.[2][3][4] This is believed to be because of activity at a side target, the 5-HT2A receptor, which is better known as the target of drugs such as LSD.[5]
Pricing information
As with most HIV treatments, efavirenz is quite expensive. A one month supply of 600 mg tablets costed approximately $550 in April 2008.[6] Some emerging countries have opted to purchase Indian generics such as Efavir by Cipla Ltd for a fraction of the cost. In Thailand, one month supply of Efavirenz + Truvada, as of June 2012, costs THB 2900 ($90), there's also a social program for poorer patients who can't afford even this price.[7] In South Africa, a license has been granted to generics giant Aspen Pharmacare to manufacture, and distribute to Sub-Saharan Africa, a cost-effective antiretroviral drug.[8]
Mechanism of Action
Efavirenz is a non-nucleoside reverse transcriptase inhibitor (NNRTI) with activity against HIV-1 by binding to reverse transcriptase. It consequently blocks the RNA-dependent and DNA-dependent DNA polymerase activities including HIV-1 replication. It does not require intracellular phosphorylation for antiviral activity.
References
- ↑ "Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents". Retrieved 10 May 2013.
- ↑ IOL: Thugs get high on stolen Aids drugs
- ↑ Getting high on HIV drugs in S Africa. BBC News, 8 December 2008.
- ↑ 'No Turning Back': Teens Abuse HIV Drugs. ABC News, April 6, 2009.
- ↑ PMID 23702798 (PMID 23702798)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Price listed on http://drugstore.com website, 4/20/2008
- ↑ IndiaDaily - A new trend in emerging nations - Brazil opts for Indian generic drug ignoring US pharmaceutical giant Merck’s patent on AIDS drug Efavirenz
- ↑ Patrick Lumumba Osewe; Yvonne Korkoi Nkrumah; Emmanuel K. Sackey (15 June 2008). Improving Access to HIV/AIDS Medicines in Africa: Trade-Related Aspects of Intellectual Property Rights (TRIPS) Flexibilities Utilization. World Bank Publications. pp. 35–39. ISBN 978-0-8213-7544-0. Retrieved 30 June 2012.