Edoxaban: Difference between revisions
Martin Nino (talk | contribs) No edit summary |
Martin Nino (talk | contribs) No edit summary |
||
| Line 5: | Line 5: | ||
|drugClass=[[factor Xa]] inhibitor | |drugClass=[[factor Xa]] inhibitor | ||
|indicationType=prevention | |indicationType=prevention | ||
|indication=[[stroke]] and [[ | |indication=[[stroke]] and systemic [[embolism]] (SE) in patients with nonvalvular [[atrial fibrillation]] ([[NVAF]]). Is also indicated for the treatment of [[deep vein thrombosis]] ([[DVT]]) and [[pulmonary embolism]] ([[PE]]) following 5 to 10 days of initial therapy with a [[parenteral]] [[anticoagulant]] | ||
|adverseReactions=[[bleeding]] and [[anemia]] in the treatment of [[NVAF]] (≥ 5%) and [[bleeding]], [[rash]], abnormal [[liver function]] tests and [[anemia]] in the treatment of [[DVT]] and [[PE]] (≥ 1%) | |adverseReactions=[[bleeding]] and [[anemia]] in the treatment of [[NVAF]] (≥ 5%) and [[bleeding]], [[rash]], abnormal [[liver function]] tests and [[anemia]] in the treatment of [[DVT]] and [[PE]] (≥ 1%) | ||
| Line 37: | Line 37: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
======Indications====== | ======Indications====== | ||
:*'''Reduction in the Risk of [[Stroke]] and Systemic [[Embolism]] in [[atrial fibrillation|Nonvalvular Atrial Fibrillation]]''' | |||
SAVAYSA is indicated to reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF). | |||
::*Limitation of Use for NVAF | |||
SAVAYSA should not be used in patients with [[creatinine clearance|CrCL]] > 95 mL/min because of an increased risk of ischemic [[stroke]] compared to [[warfarin]]. | |||
:*'''Treatment of [[Deep Vein Thrombosis]] and [[Pulmonary Embolism]]''' | |||
SAVAYSA is indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a [[parenteral]] [[anticoagulant]]. | |||
======Dosage====== | ======Dosage====== | ||
| | :*'''[[atrial fibrillation|Nonvalvular Atrial Fibrillation]]''' | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | The recommended dose of SAVAYSA is 60 mg taken orally once daily. Assess [[creatinine clearance]], as calculated using the [[Cockcroft-Gault equation]]*, before initiating therapy with SAVAYSA. Do not use SAVAYSA in patients with CrCL > 95 mL/min. | ||
|fdaLIADPed=Safety and effectiveness have not been established | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | Reduce SAVAYSA dose to 30 mg once daily in patients with CrCL 15 to 50 mL/min. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | |||
*Cockcroft-Gault CrCL = (140-age) x (weight in kg) x (0.85 if female) / (72 x creatinine in mg/dL). | |||
:*'''Treatment of [[Deep Vein Thrombosis]] and [[Pulmonary Embolism]]''' | |||
The recommended dose of SAVAYSA is 60 mg taken orally once daily following 5 to 10 days of initial therapy with a [[parenteral]] [[anticoagulant]]. | |||
The recommended dose of SAVAYSA is 30 mg once daily in patients with [[creatinine clearance|CrCL]] 15 to 50 mL/min, patients who weigh less than or equal to 60 kg, or patients who are taking certain concomitant [[P-gp]] inhibitor medications based on clinical study data in this indication. | |||
:*Administration Information | |||
If a dose of SAVAYSA is missed, the dose should be taken as soon as possible on the same day. Dosing should resume the next day according to the normal dosing schedule. The dose should not be doubled to make up for a missed dose. | |||
SAVAYSA can be taken without regard to food. | |||
:*Transition to or from SAVAYSA | |||
:*'''Transition to SAVAYSA''' | |||
[[File:edo1.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>SAVAYSA: Edoxaban tosylate's Brand name</SMALL> | |||
:*'''Transition from SAVAYSA''' | |||
[[File:edo2.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>SAVAYSA: Edoxaban tosylate's Brand name</SMALL> | |||
:*Discontinuation for Surgery and Other Interventions | |||
Discontinue SAVAYSA at least 24 hours before invasive or surgical procedures because of the risk of [[bleeding]]. | |||
If surgery cannot be delayed, there is an increased risk of bleeding. This risk of bleeding should be weighed against the urgency of intervention. | |||
SAVAYSA can be restarted after the surgical or other procedure as soon as adequate [[hemostasis]] has been established noting that the time to onset of [[pharmacodynamic]] effect is 1-2 hours. Administer a [[parenteral]] [[anticoagulant]] and then switch to oral SAVAYSA, if oral medication cannot be taken during or after surgical intervention. | |||
|offLabelAdultGuideSupport= | |||
Total [[arthroplasty]] of knee - Postoperative [[deep vein thrombosis]] (Prophylaxis)<ref name="pmid25294589">{{cite journal| author=Fuji T, Wang CJ, Fujita S, Kawai Y, Nakamura M, Kimura T et al.| title=Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial. | journal=Thromb Res | year= 2014 | volume= 134 | issue= 6 | pages= 1198-204 | pmid=25294589 | doi=10.1016/j.thromres.2014.09.011 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25294589 }} </ref> | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Edoxaban tosylate in adult patients. | |||
|fdaLIADPed=Safety and effectiveness in pediatric patients have not been established. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Edoxaban tosylate in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Edoxaban tosylate in pediatric patients. | |||
|contraindications= | |contraindications= | ||
SAVAYSA is contraindicated in patients with active pathological [[bleeding]]. | |||
|warnings= | |warnings= | ||
======Reduced Efficacy in Nonvalvular Atrial Fibrillation Patients with CrCL > 95 mL/min====== | |||
SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the randomized ENGAGE AF-TIMI 48 study, NVAF patients with CrCL > 95 mL/min had an increased rate of [[ischemic stroke]] with SAVAYSA 60 mg daily compared to patients treated with [[warfarin]]. In these patients another [[anticoagulant]] should be used. | |||
======Increased Risk of [[Stroke]] with Discontinuation of SAVAYSA in Patients with Nonvalvular Atrial Fibrillation====== | |||
Premature discontinuation of any oral [[anticoagulant]] in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If SAVAYSA is discontinued for a reason other than pathological [[bleeding]] or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance. | |||
======Risk of [[Bleeding]]====== | |||
SAVAYSA increases the risk of bleeding and can cause serious and potentially fatal bleeding. Promptly evaluate any signs or symptoms of blood loss. | |||
Discontinue SAVAYSA in patients with active pathological bleeding. | |||
Concomitant use of drugs affecting [[hemostasis]] may increase the risk of bleeding. These include [[aspirin]] and other [[antiplatelet]] agents, other [[antithrombotic]] agents, [[fibrinolytic]] therapy, chronic use of [[nonsteroidal anti-inflammatory drug]]s (NSAIDs), selective [[serotonin]] reuptake inhibitors and [[serotonin]] [[norepinephrine]] reuptake inhibitors. | |||
:*'''Reversal of Anticoagulant Effect''' | |||
There is no established way to reverse the anticoagulant effects of SAVAYSA, which can be expected to persist for approximately 24 hours after the last dose. The anticoagulant effect of SAVAYSA cannot be reliably monitored with standard laboratory testing. A specific reversal agent for edoxaban is not available. [[Hemodialysis]] does not significantly contribute to edoxaban clearance. [[Protamine sulfate]], [[vitamin K]], and [[tranexamic acid]] are not expected to reverse the anticoagulant activity of SAVAYSA. The use of [[prothrombin complex concentrates]] (PCC), or other [[procoagulant]] reversal agents such as [[activated prothrombin complex concentrate]] (APCC) or [[recombinant factor VIIa]] (rFVIIa) may be considered but has not been evaluated in clinical outcome studies. When PCCs are used, monitoring for anticoagulation effect of edoxaban using [[clotting test]] ([[PT]], [[INR]], or [[aPTT]]) or [[anti-FXa]] activity is not useful and is not recommended. | |||
======Spinal/Epidural Anesthesia or Puncture====== | |||
When neuraxial [[anesthesia]] (spinal/epidural anesthesia) or spinal/epidural [[puncture]] is employed, patients treated with [[antithrombotic]] agents for prevention of [[thromboembolic]] complications are at risk of developing an epidural or spinal [[hematoma]], which can result in long-term or permanent [[paralysis]]. | |||
The risk of these events may be increased by the postoperative use of indwelling [[epidural catheter]]s or the concomitant use of medicinal products affecting [[hemostasis]]. Indwelling [[epidural]] or [[intrathecal]] catheters should not be removed earlier than 12 hours after the last administration of SAVAYSA. The next dose of SAVAYSA should not be administered earlier than 2 hours after the removal of the [[catheter]]. The risk may also be increased by traumatic or repeated epidural or spinal puncture. | |||
Monitor patients frequently for signs and symptoms of neurological impairment (e.g., [[numbness]] or [[weakness]] of the legs, bowel, or [[bladder dysfunction]]). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for [[thromboprophylaxis]]. | |||
======Patients with [[Mechanical Heart Valves]] or Moderate to Severe [[Mitral Stenosis]]====== | |||
The safety and efficacy of SAVAYSA has not been studied in patients with mechanical heart valves or moderate to severe mitral stenosis. The use of SAVAYSA is not recommended in these patients. | |||
|clinicalTrials= | |clinicalTrials= | ||
| | The following serious adverse reactions are discussed in greater detail in other sections of the prescribing information. | ||
:*Increased risk of [[stroke]] with discontinuation of SAVAYSA in patients with NVAF | |||
:*Spinal/epidural [[anesthesia]] or [[puncture]] | |||
The most serious adverse reactions reported with SAVAYSA were related to [[bleeding]]. | |||
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
The safety of SAVAYSA was evaluated in the ENGAGE AF-TIMI 48 and Hokusai VTE studies including 11,130 patients exposed to SAVAYSA 60 mg and 7002 patients exposed to SAVAYSA 30 mg once daily. | |||
:*'''The ENGAGE AF-TIMI 48 Study''' | |||
In the ENGAGE AF-TIMI 48 study, the median study drug exposure for the SAVAYSA and [[warfarin]] treatment groups was 2.5 years. | |||
[[Bleeding]] was the most common reason for treatment discontinuation. Bleeding led to treatment discontinuation in 3.9% and 4.1% of patients in the SAVAYSA 60 mg and warfarin treatment groups, respectively. | |||
In the overall population, Major Bleeding was lower in the SAVAYSA group compared to the warfarin group [HR 0.80 (0.70, 0.91), p<0.001]. TABLE 1 shows Major Bleeding events (percentage of patients with at least one bleeding event, per year) for the indicated population (CrCL ≤ 95 mL/min). | |||
:*'''Table 1: Adjudicated Bleeding Events for NVAF Patients with CrCL ≤ 95 mL/min*''' | |||
[[File:table1_edo.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>SAVAYSA: Edoxaban tosylate's Brand name</SMALL> | |||
The most common site of a Major Bleeding event was the gastrointestinal (GI) tract. TABLE 2 shows the number of and the rate at which patients experienced GI bleeding in the SAVAYSA 60 mg and [[warfarin]] treatment groups. | |||
:*'''Table 2: Gastrointestinal Bleeding Events for NVAF Patients with CrCL ≤ 95 mL/min*''' | |||
[[File:table2_edo.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>SAVAYSA: Edoxaban tosylate's Brand name</SMALL> | |||
The rate of [[anemia]]-related adverse events was greater with SAVAYSA 60 mg than with [[warfarin]] (9.6% vs. 6.8%). | |||
The comparative rates of Major Bleeding on SAVAYSA and warfarin were generally consistent among subgroups (see FIGURE 1). Bleeding rates appeared higher in both treatment arms (SAVAYSA and warfarin) in the following subgroups of patients: those receiving [[aspirin]], those in the United States, those more than 75 years old and those with reduced [[renal function]]. | |||
:*'''Figure 1: Adjudicated Major Bleeding in the ENGAGE AF-TIMI 48* Study''' | |||
[[File:figure1_edo.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>SAVAYSA: Edoxaban tosylate's Brand name</SMALL> | |||
:*'''Other Adverse Reactions''' | |||
The most common non-bleeding adverse reactions (≥ 1%) for SAVAYSA 60 mg versus [[warfarin]] were [[rash]] (4.2% vs. 4.1%), and abnormal [[liver function]] tests (4.8% vs. 4.6%), respectively. | |||
[[Interstitial Lung Disease]] (ILD) was reported as a serious adverse event on treatment for SAVAYSA 60 mg and [[warfarin]] in 15 (0.2%) and 7 (0.1%) patients, respectively. Many of the cases in both treatment groups were confounded by the use of [[amiodarone]], which has been associated with ILD, or by infectious [[pneumonia]]. In the overall study period, there were 5 and 0 fatal ILD cases in the SAVAYSA 60 mg and warfarin groups, respectively. | |||
:*'''The Hokusai VTE Study''' | |||
In the Hokusai VTE study, the duration of drug exposure for SAVAYSA was ≤ 6 months for 1561 (37.9%) of patients, > 6 months for 2557 (62.1%) of patients and 12 months for 1661 (40.3%) of patients. | |||
[[Bleeding]] was the most common reason for treatment discontinuation and occurred in 1.4% and 1.4% of patients in the SAVAYSA and [[warfarin]] arms, respectively. | |||
:*'''[[Bleeding]] in Patients with [[DVT]] and/or [[PE]] in the Hokusai VTE Study''' | |||
The primary safety endpoint was Clinically Relevant Bleeding, defined as the composite of Major and Clinically Relevant Non-Major (CRNM) Bleeding that occurred during or within three days of stopping study treatment. The incidence of Clinically Relevant Bleeding was lower in SAVAYSA than warfarin [HR (95% CI): 0.81 (0.71, 0.94); p =0.004]. | |||
TABLE 3 shows the number of patients experiencing bleeding events in the Hokusai VTE Study. | |||
:*'''Table 3: Bleeding Events in the Hokusai VTE Study''' | |||
[[File:table3_edo.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>SAVAYSA: Edoxaban tosylate's Brand name</SMALL> | |||
Patients with low body weight (≤ 60 kg), CrCL ≤ 50 mL/min, or concomitant use of select [[P-gp]] inhibitors were randomized to receive SAVAYSA 30 mg or [[warfarin]]. As compared to all patients who received SAVAYSA or warfarin in the 60 mg [[cohort]], all patients who received SAVAYSA or warfarin in the 30 mg cohort (n= 1452, 17.6% of the entire study population) were older (60.1 vs 54.9 years), more frequently female (66.5% vs 37.7%), more frequently of Asian race (46.0% vs 15.6%) and had more co-morbidities (e.g., history of bleeding, [[hypertension]], [[diabetes]], [[cardiovascular disease]], [[cancer]]). Clinically relevant bleeding events occurred in 58/733 (7.9%) of the SAVAYSA patients receiving 30 mg once daily and 92/719 (12.8%) of warfarin patients meeting the above criteria. | |||
In the Hokusai VTE study, among all patients the most common bleeding adverse reactions (≥ 1%) are shown in TABLE 4. | |||
:*'''Table 4: Adverse Reactions Occurring in ≥ 1% of Patients Treated in Hokusai VTE''' | |||
[[File:table4_edo.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>SAVAYSA: Edoxaban tosylate's Brand name</SMALL> | |||
|drugInteractions= | |drugInteractions= | ||
|FDAPregCat= | :*'''[[Anticoagulants]], [[Antiplatelets]], and [[Thrombolytics]]''' | ||
Co-administration of anticoagulants, antiplatelet drugs, and thrombolytics may increase the risk of [[bleeding]]. Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with anticoagulants, [[aspirin]], other [[platelet aggregation]] inhibitors, and/or [[NSAIDs]]. | |||
Long-term concomitant treatment with SAVAYSA and other anticoagulants is not recommended because of increased risk of bleeding. Short term co-administration may be needed for patients transitioning to or from SAVAYSA. | |||
In clinical studies with SAVAYSA concomitant use of [[aspirin]] (low dose ≤ 100 mg/day) or [[thienopyridines]], and [[NSAIDs]] was permitted and resulted in increased rates of Clinically Relevant Bleeding. Carefully monitor for bleeding in patients who require chronic treatment with low dose aspirin and/or NSAIDs. | |||
:*'''[[P-gp]] Inducers''' | |||
Avoid the concomitant use of SAVAYSA with [[rifampin]]. | |||
:*'''[[P-gp]] Inhibitors''' | |||
::*Treatment of NVAF | |||
Based on clinical experience from the ENGAGE AF-TIMI 48 study, dose reduction in patients concomitantly receiving P-gp inhibitors resulted in edoxaban blood levels that were lower than in patients who were given the full dose. Consequently, no dose reduction is recommended for concomitant P-gp inhibitor use. | |||
::*Treatment of [[Deep Vein Thrombosis]] and [[Pulmonary Embolism]] | |||
<i>See Clinical Studies</i> | |||
|FDAPregCat=C | |||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
:*Risk Summary | |||
There are no adequate and well-controlled studies in pregnant women. SAVAYSA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
:*Human Data | |||
In the Hokusai VTE study there were 10 pregnancy cases reported in patients receiving SAVAYSA with exposure in the first trimester and estimated duration of exposure for up to approximately 6 weeks. Among these there were 6 live births (4 full term, 2 pre-term), 1 first-trimester [[spontaneous abortion]], and 3 cases of elective termination of pregnancy. | |||
:*Animal Data | |||
Embryo-fetal development studies were conducted in pregnant rats and rabbits during the period of [[organogenesis]]. In rats, no [[teratogenic]] effects were seen when edoxaban was administered orally at doses up to 300 mg/kg/day, or 49 times the human dose of 60 mg/day normalized to [[body surface area]]. Increased post-implantation loss occurred at 300 mg/kg/day, but this effect may be secondary to the maternal vaginal [[hemorrhage]] seen at this dose. In rabbits, no teratogenic effects were seen at doses up to 600 mg/kg/day (49 times the human exposure at a dose of 60 mg/day when based on [[AUC]]). Embryo-fetal toxicities occurred at maternally toxic doses, and included absent or small fetal [[gallbladder]] at 600 mg/kg/day, and increased post-implantation loss, increased [[spontaneous abortion]], and decreased live fetuses and fetal weight at doses equal to or greater than 200 mg/kg/day, which is equal to or greater than 20 times the human exposure. | |||
In a rat pre- and post-natal developmental study, edoxaban was administered orally during the period of [[organogenesis]] and through lactation day 20 at doses up to 30 mg/kg/day, which is up to 3 times the human exposure when based on [[AUC]]. Vaginal bleeding in pregnant rats and delayed avoidance response (a learning test) in female offspring were seen at 30 mg/kg/day. | |||
|useInLaborDelivery= | |useInLaborDelivery= | ||
|useInNursing= | Safety and effectiveness of SAVAYSA during labor and delivery have not been studied in clinical studies. The risks of [[bleeding]] should be balanced with the risk of [[thrombotic]] events when considering the use of SAVAYSA in this setting. | ||
|useInPed= | |||
|useInGeri= | |useInNursing=It is not known if edoxaban is excreted in human milk. Edoxaban was excreted in the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from SAVAYSA, a decision should be made to discontinue nursing or the drug, taking into account the importance of the drug to the mother. | ||
|useInRenalImpair= | |useInPed=Safety and effectiveness in pediatric patients have not been established. | ||
|useInHepaticImpair= | |useInGeri=Of the total patients in the ENGAGE AF-TIMI 48 study, 5182 (74%) were 65 years and older, while 2838 (41%) were 75 years and older. In Hokusai VTE, 1334 (32%) patients were 65 years and older, while 560 (14%) patients were 75 years and older. In clinical trials the efficacy and safety of SAVAYSA in elderly (65 years or older) and younger patients were similar. | ||
|useInRenalImpair=Renal [[clearance]] accounts for approximately 50% of the total clearance of edoxaban. Consequently, edoxaban blood levels are increased in patients with poor [[renal function]] compared to those with higher renal function. Reduce SAVAYSA dose to 30 mg once daily in patients with CrCL 15-50 mL/min. There are limited clinical data with SAVAYSA in patients with CrCL < 15 mL/min; SAVAYSA is therefore not recommended in these patients. [[Hemodialysis]] does not significantly contribute to SAVAYSA clearance. | |||
|othersTitle= | As [[renal function]] improves and edoxaban blood levels decrease, the risk for [[ischemic stroke]] increases in patients with NVAF. | ||
|useInOthers= | |||
|useInHepaticImpair=The use of SAVAYSA in patients with moderate or severe hepatic impairment ([[Child-Pugh]] B and C) is not recommended as these patients may have [[intrinsic coagulation]] abnormalities. No dose reduction is required in patients with mild hepatic impairment (Child-Pugh A). | |||
|othersTitle=Low Body Weight Consideration for Patients treated for DVT and/or PE | |||
|useInOthers=Based on the clinical experience from the Hokusai VTE study, reduce SAVAYSA dose to 30 mg in patients with body weight less than or equal to 60 kg. | |||
|overdose= | |overdose= | ||
A specific reversal agent for edoxaban is not available. Overdose of SAVAYSA increases the risk of [[bleeding]]. | |||
The following are not expected to reverse the anticoagulant effects of edoxaban: [[protamine sulfate]], [[vitamin K]], and [[tranexamic acid]]. | |||
[[Hemodialysis]] does not significantly contribute to edoxaban clearance. | |||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
}} | }} | ||
| Line 72: | Line 261: | ||
|clinicalStudies= | |clinicalStudies= | ||
|howSupplied= | |howSupplied= | ||
60 mg, yellow round shaped, film-coated tablets, debossed with DSC L60 on one side | |||
30 mg, pink round shaped, film-coated tablets, debossed with DSC L30 on one side | |||
15 mg, orange round shaped, film-coated tablets, debossed with DSC L15 on one side | |||
|storage= | |storage= | ||
|packLabel=[[File:pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |packLabel=[[File:pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
Revision as of 17:36, 7 February 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
(A) REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CREATININE CLEARANCE (CRCL) > 95 ML/MIN (B) PREMATURE DISCONTINUATION OF EDOXABAN TOSYLATE INCREASES THE RISK OF ISCHEMIC EVENTS (C) SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete Boxed Warning.
A. REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CRCL > 95 ML/MIN SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the ENGAGE AF-TIMI 48 study, nonvalvular atrial fibrillation patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with SAVAYSA 60 mg once daily compared to patients treated with warfarin. In these patients another anticoagulant should be used. B. PREMATURE DISCONTINUATION OF EDOXABAN TOSYLATE INCREASES THE RISK OF ISCHEMIC EVENTS Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If SAVAYSA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance. C. SPINAL/EPIDURAL HEMATOMA Epidural or spinal hematomas may occur in patients treated with SAVAYSA who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated. |
Overview
Edoxaban is a factor Xa inhibitor that is FDA approved for the prevention of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF). Is also indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a parenteral anticoagulant. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bleeding and anemia in the treatment of NVAF (≥ 5%) and bleeding, rash, abnormal liver function tests and anemia in the treatment of DVT and PE (≥ 1%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Reduction in the Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
SAVAYSA is indicated to reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF).
- Limitation of Use for NVAF
SAVAYSA should not be used in patients with CrCL > 95 mL/min because of an increased risk of ischemic stroke compared to warfarin.
- Treatment of Deep Vein Thrombosis and Pulmonary Embolism
SAVAYSA is indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a parenteral anticoagulant.
Dosage
The recommended dose of SAVAYSA is 60 mg taken orally once daily. Assess creatinine clearance, as calculated using the Cockcroft-Gault equation*, before initiating therapy with SAVAYSA. Do not use SAVAYSA in patients with CrCL > 95 mL/min.
Reduce SAVAYSA dose to 30 mg once daily in patients with CrCL 15 to 50 mL/min.
*Cockcroft-Gault CrCL = (140-age) x (weight in kg) x (0.85 if female) / (72 x creatinine in mg/dL).
- Treatment of Deep Vein Thrombosis and Pulmonary Embolism
The recommended dose of SAVAYSA is 60 mg taken orally once daily following 5 to 10 days of initial therapy with a parenteral anticoagulant.
The recommended dose of SAVAYSA is 30 mg once daily in patients with CrCL 15 to 50 mL/min, patients who weigh less than or equal to 60 kg, or patients who are taking certain concomitant P-gp inhibitor medications based on clinical study data in this indication.
- Administration Information
If a dose of SAVAYSA is missed, the dose should be taken as soon as possible on the same day. Dosing should resume the next day according to the normal dosing schedule. The dose should not be doubled to make up for a missed dose.
SAVAYSA can be taken without regard to food.
- Transition to or from SAVAYSA
- Transition to SAVAYSA

SAVAYSA: Edoxaban tosylate's Brand name
- Transition from SAVAYSA
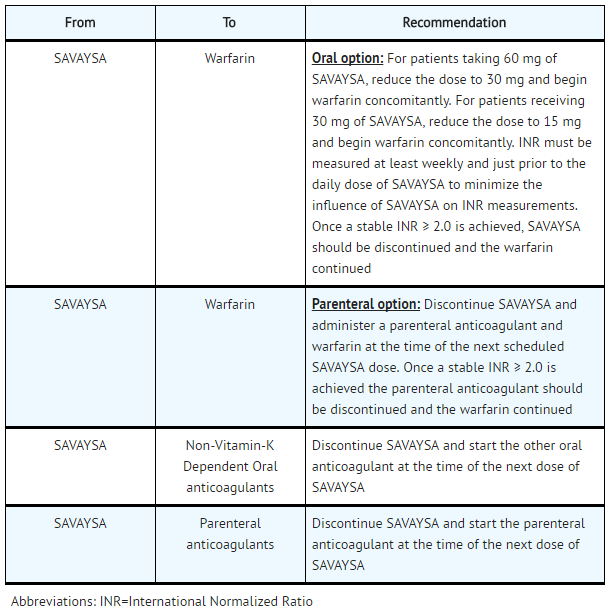
SAVAYSA: Edoxaban tosylate's Brand name
- Discontinuation for Surgery and Other Interventions
Discontinue SAVAYSA at least 24 hours before invasive or surgical procedures because of the risk of bleeding.
If surgery cannot be delayed, there is an increased risk of bleeding. This risk of bleeding should be weighed against the urgency of intervention.
SAVAYSA can be restarted after the surgical or other procedure as soon as adequate hemostasis has been established noting that the time to onset of pharmacodynamic effect is 1-2 hours. Administer a parenteral anticoagulant and then switch to oral SAVAYSA, if oral medication cannot be taken during or after surgical intervention.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Total arthroplasty of knee - Postoperative deep vein thrombosis (Prophylaxis)[1]
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Edoxaban tosylate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Edoxaban tosylate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Edoxaban tosylate in pediatric patients.
Contraindications
SAVAYSA is contraindicated in patients with active pathological bleeding.
Warnings
|
(A) REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CREATININE CLEARANCE (CRCL) > 95 ML/MIN (B) PREMATURE DISCONTINUATION OF EDOXABAN TOSYLATE INCREASES THE RISK OF ISCHEMIC EVENTS (C) SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete Boxed Warning.
A. REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CRCL > 95 ML/MIN SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the ENGAGE AF-TIMI 48 study, nonvalvular atrial fibrillation patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with SAVAYSA 60 mg once daily compared to patients treated with warfarin. In these patients another anticoagulant should be used. B. PREMATURE DISCONTINUATION OF EDOXABAN TOSYLATE INCREASES THE RISK OF ISCHEMIC EVENTS Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If SAVAYSA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance. C. SPINAL/EPIDURAL HEMATOMA Epidural or spinal hematomas may occur in patients treated with SAVAYSA who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated. |
Reduced Efficacy in Nonvalvular Atrial Fibrillation Patients with CrCL > 95 mL/min
SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the randomized ENGAGE AF-TIMI 48 study, NVAF patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with SAVAYSA 60 mg daily compared to patients treated with warfarin. In these patients another anticoagulant should be used.
Increased Risk of Stroke with Discontinuation of SAVAYSA in Patients with Nonvalvular Atrial Fibrillation
Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If SAVAYSA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance.
Risk of Bleeding
SAVAYSA increases the risk of bleeding and can cause serious and potentially fatal bleeding. Promptly evaluate any signs or symptoms of blood loss.
Discontinue SAVAYSA in patients with active pathological bleeding.
Concomitant use of drugs affecting hemostasis may increase the risk of bleeding. These include aspirin and other antiplatelet agents, other antithrombotic agents, fibrinolytic therapy, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors.
- Reversal of Anticoagulant Effect
There is no established way to reverse the anticoagulant effects of SAVAYSA, which can be expected to persist for approximately 24 hours after the last dose. The anticoagulant effect of SAVAYSA cannot be reliably monitored with standard laboratory testing. A specific reversal agent for edoxaban is not available. Hemodialysis does not significantly contribute to edoxaban clearance. Protamine sulfate, vitamin K, and tranexamic acid are not expected to reverse the anticoagulant activity of SAVAYSA. The use of prothrombin complex concentrates (PCC), or other procoagulant reversal agents such as activated prothrombin complex concentrate (APCC) or recombinant factor VIIa (rFVIIa) may be considered but has not been evaluated in clinical outcome studies. When PCCs are used, monitoring for anticoagulation effect of edoxaban using clotting test (PT, INR, or aPTT) or anti-FXa activity is not useful and is not recommended.
Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma, which can result in long-term or permanent paralysis.
The risk of these events may be increased by the postoperative use of indwelling epidural catheters or the concomitant use of medicinal products affecting hemostasis. Indwelling epidural or intrathecal catheters should not be removed earlier than 12 hours after the last administration of SAVAYSA. The next dose of SAVAYSA should not be administered earlier than 2 hours after the removal of the catheter. The risk may also be increased by traumatic or repeated epidural or spinal puncture.
Monitor patients frequently for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, bowel, or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
Patients with Mechanical Heart Valves or Moderate to Severe Mitral Stenosis
The safety and efficacy of SAVAYSA has not been studied in patients with mechanical heart valves or moderate to severe mitral stenosis. The use of SAVAYSA is not recommended in these patients.
Adverse Reactions
Clinical Trials Experience
The following serious adverse reactions are discussed in greater detail in other sections of the prescribing information.
- Increased risk of stroke with discontinuation of SAVAYSA in patients with NVAF
- Spinal/epidural anesthesia or puncture
The most serious adverse reactions reported with SAVAYSA were related to bleeding.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SAVAYSA was evaluated in the ENGAGE AF-TIMI 48 and Hokusai VTE studies including 11,130 patients exposed to SAVAYSA 60 mg and 7002 patients exposed to SAVAYSA 30 mg once daily.
- The ENGAGE AF-TIMI 48 Study
In the ENGAGE AF-TIMI 48 study, the median study drug exposure for the SAVAYSA and warfarin treatment groups was 2.5 years.
Bleeding was the most common reason for treatment discontinuation. Bleeding led to treatment discontinuation in 3.9% and 4.1% of patients in the SAVAYSA 60 mg and warfarin treatment groups, respectively.
In the overall population, Major Bleeding was lower in the SAVAYSA group compared to the warfarin group [HR 0.80 (0.70, 0.91), p<0.001]. TABLE 1 shows Major Bleeding events (percentage of patients with at least one bleeding event, per year) for the indicated population (CrCL ≤ 95 mL/min).
- Table 1: Adjudicated Bleeding Events for NVAF Patients with CrCL ≤ 95 mL/min*
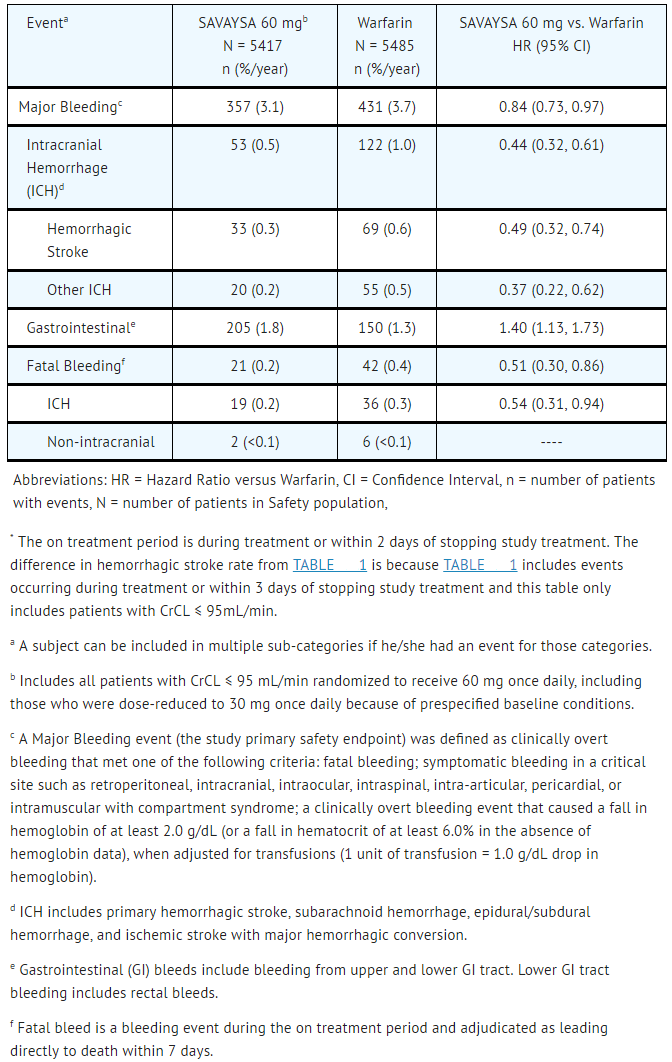
SAVAYSA: Edoxaban tosylate's Brand name
The most common site of a Major Bleeding event was the gastrointestinal (GI) tract. TABLE 2 shows the number of and the rate at which patients experienced GI bleeding in the SAVAYSA 60 mg and warfarin treatment groups.
- Table 2: Gastrointestinal Bleeding Events for NVAF Patients with CrCL ≤ 95 mL/min*

SAVAYSA: Edoxaban tosylate's Brand name
The rate of anemia-related adverse events was greater with SAVAYSA 60 mg than with warfarin (9.6% vs. 6.8%).
The comparative rates of Major Bleeding on SAVAYSA and warfarin were generally consistent among subgroups (see FIGURE 1). Bleeding rates appeared higher in both treatment arms (SAVAYSA and warfarin) in the following subgroups of patients: those receiving aspirin, those in the United States, those more than 75 years old and those with reduced renal function.
- Figure 1: Adjudicated Major Bleeding in the ENGAGE AF-TIMI 48* Study

SAVAYSA: Edoxaban tosylate's Brand name
- Other Adverse Reactions
The most common non-bleeding adverse reactions (≥ 1%) for SAVAYSA 60 mg versus warfarin were rash (4.2% vs. 4.1%), and abnormal liver function tests (4.8% vs. 4.6%), respectively.
Interstitial Lung Disease (ILD) was reported as a serious adverse event on treatment for SAVAYSA 60 mg and warfarin in 15 (0.2%) and 7 (0.1%) patients, respectively. Many of the cases in both treatment groups were confounded by the use of amiodarone, which has been associated with ILD, or by infectious pneumonia. In the overall study period, there were 5 and 0 fatal ILD cases in the SAVAYSA 60 mg and warfarin groups, respectively.
- The Hokusai VTE Study
In the Hokusai VTE study, the duration of drug exposure for SAVAYSA was ≤ 6 months for 1561 (37.9%) of patients, > 6 months for 2557 (62.1%) of patients and 12 months for 1661 (40.3%) of patients.
Bleeding was the most common reason for treatment discontinuation and occurred in 1.4% and 1.4% of patients in the SAVAYSA and warfarin arms, respectively.
The primary safety endpoint was Clinically Relevant Bleeding, defined as the composite of Major and Clinically Relevant Non-Major (CRNM) Bleeding that occurred during or within three days of stopping study treatment. The incidence of Clinically Relevant Bleeding was lower in SAVAYSA than warfarin [HR (95% CI): 0.81 (0.71, 0.94); p =0.004].
TABLE 3 shows the number of patients experiencing bleeding events in the Hokusai VTE Study.
- Table 3: Bleeding Events in the Hokusai VTE Study

SAVAYSA: Edoxaban tosylate's Brand name
Patients with low body weight (≤ 60 kg), CrCL ≤ 50 mL/min, or concomitant use of select P-gp inhibitors were randomized to receive SAVAYSA 30 mg or warfarin. As compared to all patients who received SAVAYSA or warfarin in the 60 mg cohort, all patients who received SAVAYSA or warfarin in the 30 mg cohort (n= 1452, 17.6% of the entire study population) were older (60.1 vs 54.9 years), more frequently female (66.5% vs 37.7%), more frequently of Asian race (46.0% vs 15.6%) and had more co-morbidities (e.g., history of bleeding, hypertension, diabetes, cardiovascular disease, cancer). Clinically relevant bleeding events occurred in 58/733 (7.9%) of the SAVAYSA patients receiving 30 mg once daily and 92/719 (12.8%) of warfarin patients meeting the above criteria.
In the Hokusai VTE study, among all patients the most common bleeding adverse reactions (≥ 1%) are shown in TABLE 4.
- Table 4: Adverse Reactions Occurring in ≥ 1% of Patients Treated in Hokusai VTE
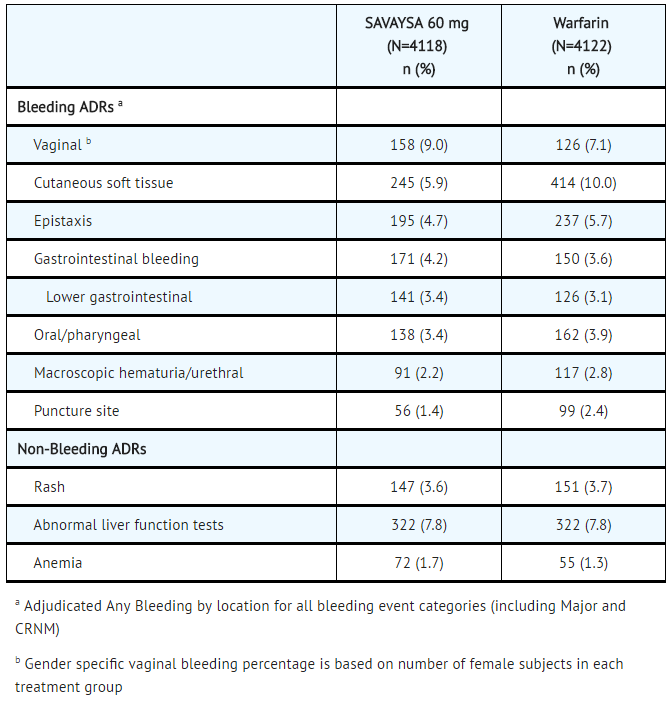
SAVAYSA: Edoxaban tosylate's Brand name
Postmarketing Experience
There is limited information regarding Edoxaban Postmarketing Experience in the drug label.
Drug Interactions
Co-administration of anticoagulants, antiplatelet drugs, and thrombolytics may increase the risk of bleeding. Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with anticoagulants, aspirin, other platelet aggregation inhibitors, and/or NSAIDs.
Long-term concomitant treatment with SAVAYSA and other anticoagulants is not recommended because of increased risk of bleeding. Short term co-administration may be needed for patients transitioning to or from SAVAYSA.
In clinical studies with SAVAYSA concomitant use of aspirin (low dose ≤ 100 mg/day) or thienopyridines, and NSAIDs was permitted and resulted in increased rates of Clinically Relevant Bleeding. Carefully monitor for bleeding in patients who require chronic treatment with low dose aspirin and/or NSAIDs.
- P-gp Inducers
Avoid the concomitant use of SAVAYSA with rifampin.
- P-gp Inhibitors
- Treatment of NVAF
Based on clinical experience from the ENGAGE AF-TIMI 48 study, dose reduction in patients concomitantly receiving P-gp inhibitors resulted in edoxaban blood levels that were lower than in patients who were given the full dose. Consequently, no dose reduction is recommended for concomitant P-gp inhibitor use.
- Treatment of Deep Vein Thrombosis and Pulmonary Embolism
See Clinical Studies
Use in Specific Populations
Pregnancy
- Risk Summary
There are no adequate and well-controlled studies in pregnant women. SAVAYSA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Human Data
In the Hokusai VTE study there were 10 pregnancy cases reported in patients receiving SAVAYSA with exposure in the first trimester and estimated duration of exposure for up to approximately 6 weeks. Among these there were 6 live births (4 full term, 2 pre-term), 1 first-trimester spontaneous abortion, and 3 cases of elective termination of pregnancy.
- Animal Data
Embryo-fetal development studies were conducted in pregnant rats and rabbits during the period of organogenesis. In rats, no teratogenic effects were seen when edoxaban was administered orally at doses up to 300 mg/kg/day, or 49 times the human dose of 60 mg/day normalized to body surface area. Increased post-implantation loss occurred at 300 mg/kg/day, but this effect may be secondary to the maternal vaginal hemorrhage seen at this dose. In rabbits, no teratogenic effects were seen at doses up to 600 mg/kg/day (49 times the human exposure at a dose of 60 mg/day when based on AUC). Embryo-fetal toxicities occurred at maternally toxic doses, and included absent or small fetal gallbladder at 600 mg/kg/day, and increased post-implantation loss, increased spontaneous abortion, and decreased live fetuses and fetal weight at doses equal to or greater than 200 mg/kg/day, which is equal to or greater than 20 times the human exposure.
In a rat pre- and post-natal developmental study, edoxaban was administered orally during the period of organogenesis and through lactation day 20 at doses up to 30 mg/kg/day, which is up to 3 times the human exposure when based on AUC. Vaginal bleeding in pregnant rats and delayed avoidance response (a learning test) in female offspring were seen at 30 mg/kg/day.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Edoxaban in women who are pregnant.
Labor and Delivery
Safety and effectiveness of SAVAYSA during labor and delivery have not been studied in clinical studies. The risks of bleeding should be balanced with the risk of thrombotic events when considering the use of SAVAYSA in this setting.
Nursing Mothers
It is not known if edoxaban is excreted in human milk. Edoxaban was excreted in the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from SAVAYSA, a decision should be made to discontinue nursing or the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
Of the total patients in the ENGAGE AF-TIMI 48 study, 5182 (74%) were 65 years and older, while 2838 (41%) were 75 years and older. In Hokusai VTE, 1334 (32%) patients were 65 years and older, while 560 (14%) patients were 75 years and older. In clinical trials the efficacy and safety of SAVAYSA in elderly (65 years or older) and younger patients were similar.
Gender
There is no FDA guidance on the use of Edoxaban with respect to specific gender populations.
Race
There is no FDA guidance on the use of Edoxaban with respect to specific racial populations.
Renal Impairment
Renal clearance accounts for approximately 50% of the total clearance of edoxaban. Consequently, edoxaban blood levels are increased in patients with poor renal function compared to those with higher renal function. Reduce SAVAYSA dose to 30 mg once daily in patients with CrCL 15-50 mL/min. There are limited clinical data with SAVAYSA in patients with CrCL < 15 mL/min; SAVAYSA is therefore not recommended in these patients. Hemodialysis does not significantly contribute to SAVAYSA clearance.
As renal function improves and edoxaban blood levels decrease, the risk for ischemic stroke increases in patients with NVAF.
Hepatic Impairment
The use of SAVAYSA in patients with moderate or severe hepatic impairment (Child-Pugh B and C) is not recommended as these patients may have intrinsic coagulation abnormalities. No dose reduction is required in patients with mild hepatic impairment (Child-Pugh A).
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Edoxaban in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Edoxaban in patients who are immunocompromised.
Low Body Weight Consideration for Patients treated for DVT and/or PE
Based on the clinical experience from the Hokusai VTE study, reduce SAVAYSA dose to 30 mg in patients with body weight less than or equal to 60 kg.
Administration and Monitoring
Administration
There is limited information regarding Edoxaban Administration in the drug label.
Monitoring
There is limited information regarding Edoxaban Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Edoxaban and IV administrations.
Overdosage
A specific reversal agent for edoxaban is not available. Overdose of SAVAYSA increases the risk of bleeding.
The following are not expected to reverse the anticoagulant effects of edoxaban: protamine sulfate, vitamin K, and tranexamic acid.
Hemodialysis does not significantly contribute to edoxaban clearance.
Pharmacology

| |
Edoxaban
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
There is limited information regarding Edoxaban Mechanism of Action in the drug label.
Structure
There is limited information regarding Edoxaban Structure in the drug label.
Pharmacodynamics
There is limited information regarding Edoxaban Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Edoxaban Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Clinical Studies
There is limited information regarding Edoxaban Clinical Studies in the drug label.
How Supplied
60 mg, yellow round shaped, film-coated tablets, debossed with DSC L60 on one side 30 mg, pink round shaped, film-coated tablets, debossed with DSC L30 on one side 15 mg, orange round shaped, film-coated tablets, debossed with DSC L15 on one side
Storage
There is limited information regarding Edoxaban Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Edoxaban |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Edoxaban |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Edoxaban Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Edoxaban interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Edoxaban Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Edoxaban Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Fuji T, Wang CJ, Fujita S, Kawai Y, Nakamura M, Kimura T; et al. (2014). "Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial". Thromb Res. 134 (6): 1198–204. doi:10.1016/j.thromres.2014.09.011. PMID 25294589.