Clinical classification of acute myocardial infarction: Difference between revisions
Brian Blank (talk | contribs) No edit summary |
Brian Blank (talk | contribs) No edit summary |
||
| Line 204: | Line 204: | ||
==Echocardiography== | ==Echocardiography== | ||
[[Echocardiography]] is a reliable real time imaging technique with moderate spatial and temporal resolution. Its strength is the assessment of myocardial thickness, thickening, and motion at rest. This can be aided by tissue Doppler imaging. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation J | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref>Korosoglou G, Labadze N, Hansen A, | [[Echocardiography]] is a reliable real time imaging technique with moderate spatial and temporal resolution. Its strength is the assessment of myocardial thickness, thickening, and motion at rest. This can be aided by tissue Doppler imaging. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation J | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref><ref name="pmid15541235">{{cite journal |author=Korosoglou G, Labadze N, Hansen A, ''et al'' |title=Usefulness of real-time myocardial perfusion imaging in the evaluation of patients with first time chest pain |journal=Am. J. Cardiol. |volume=94 |issue=10 |pages=1225–31 |year=2004 |month=November |pmid=15541235 |doi=10.1016/j.amjcard.2004.07.104 |url=}}</ref> | ||
==Radionuclide Imaging== | ==Radionuclide Imaging== | ||
Several radionuclide tracers allow viable myocytes to be imaged directly, including thallium-201, technetium-99m MIBI, tetrofosmin, and [18F]2-fluorodeoxyglucose (FDG).<ref>Medrano R, Lowry RW, Young JB, | Several radionuclide tracers allow viable myocytes to be imaged directly, including thallium-201, technetium-99m MIBI, tetrofosmin, and [18F]2-fluorodeoxyglucose (FDG).<ref name="pmid8790039">{{cite journal |author=Medrano R, Lowry RW, Young JB, ''et al'' |title=Assessment of myocardial viability with 99mTc sestamibi in patients undergoing cardiac transplantation. A scintigraphic/pathological study |journal=Circulation |volume=94 |issue=5 |pages=1010–7 |year=1996 |month=September |pmid=8790039 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=8790039}}</ref><ref name="pmid9386154">{{cite journal |author=Dakik HA, Howell JF, Lawrie GM, ''et al'' |title=Assessment of myocardial viability with 99mTc-sestamibi tomography before coronary bypass graft surgery: correlation with histopathology and postoperative improvement in cardiac function |journal=Circulation |volume=96 |issue=9 |pages=2892–8 |year=1997 |month=November |pmid=9386154 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=9386154}}</ref><ref name="pmid11790695">{{cite journal |author=Klein C, Nekolla SG, Bengel FM, ''et al'' |title=Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography |journal=Circulation |volume=105 |issue=2 |pages=162–7 |year=2002 |month=January |pmid=11790695 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=11790695}}</ref> | ||
Radionuclide assessment of perfusion at the time of patient presentation can be performed with immediate tracer injection and imaging that can be delayed for up to several hours. The technique is interpreter dependent, although objective quantitative analysis is available. [[EKG]] gating provides simultaneous information and reliable assessment on myocardial motion, thickening, and global [[left ventricle|left ventricular]] function. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation J | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref <ref>Wagner A, Mahrholdt H, Holly TA, | Radionuclide assessment of perfusion at the time of patient presentation can be performed with immediate tracer injection and imaging that can be delayed for up to several hours. The technique is interpreter dependent, although objective quantitative analysis is available. [[EKG]] gating provides simultaneous information and reliable assessment on myocardial motion, thickening, and global [[left ventricle|left ventricular]] function. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation J | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref><ref name="pmid12573373">{{cite journal |author=Wagner A, Mahrholdt H, Holly TA, ''et al'' |title=Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study |journal=Lancet |volume=361 |issue=9355 |pages=374–9 |year=2003 |month=February |pmid=12573373 |doi=10.1016/S0140-6736(03)12389-6 |url=}}</ref> | ||
==Multi Slice Computed Tomography== | ==Multi Slice Computed Tomography== | ||
Infarcted myocardium is initially visible to computerized tomography as a focal area of decreased left ventricular enhancement, but later examination shows hyperenhancement as with late gadolinium imaging by MRI. Contrast enhanced CT may be performed and helpful for suspected embolism and aortic dissection, conditions with clinical features that overlap with those of acute myocardial infarction. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref>Gosalia A, Haramati LB, Sheth MP, Spindola-Franco H | Infarcted myocardium is initially visible to computerized tomography as a focal area of decreased left ventricular enhancement, but later examination shows hyperenhancement as with late gadolinium imaging by MRI. Contrast enhanced CT may be performed and helpful for suspected embolism and aortic dissection, conditions with clinical features that overlap with those of acute myocardial infarction. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref name="pmid15150010">{{cite journal |author=Gosalia A, Haramati LB, Sheth MP, Spindola-Franco H |title=CT detection of acute myocardial infarction |journal=AJR Am J Roentgenol |volume=182 |issue=6 |pages=1563–6 |year=2004 |month=June |pmid=15150010 |doi= |url=http://www.ajronline.org/cgi/pmidlookup?view=long&pmid=15150010}}</ref> | ||
==Cardiac Magnetic Resonance Imaging== | ==Cardiac Magnetic Resonance Imaging== | ||
| Line 220: | Line 220: | ||
Cardiac [[MRI]] (C-MRI) has high spatial resolution and moderate temporal resolution. Although not widely available and not commonly used in acute setting, it is a well validated standard for the assessment of myocardial function and has, in theory, similar capability to echocardiography in suspected acute myocardial infarction and can play an important role in its detection. | Cardiac [[MRI]] (C-MRI) has high spatial resolution and moderate temporal resolution. Although not widely available and not commonly used in acute setting, it is a well validated standard for the assessment of myocardial function and has, in theory, similar capability to echocardiography in suspected acute myocardial infarction and can play an important role in its detection. | ||
Paramagnetic contrast agents can be used to assess myocardial perfusion and the increase in extracellular space associated with the fibrosis of chronic phase of myocardial infarction.<ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref>Lima | Paramagnetic contrast agents can be used to assess myocardial perfusion and the increase in extracellular space associated with the fibrosis of chronic phase of myocardial infarction.<ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref><ref name="pmid12957440">{{cite journal |author=Lima JA |title=Myocardial viability assessment by contrast-enhanced magnetic resonance imaging |journal=J. Am. Coll. Cardiol. |volume=42 |issue=5 |pages=902–4 |year=2003 |month=September |pmid=12957440 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S0735109703008398}}</ref><ref name="pmid15944538">{{cite journal |author=Isbell DC, Kramer CM |title=Cardiovascular magnetic resonance: structure, function, perfusion, and viability |journal=J Nucl Cardiol |volume=12 |issue=3 |pages=324–36 |year=2005 |pmid=15944538 |doi= |url=}}</ref> | ||
==Diagnosis of Recurrent Myocardial Infarction (re-MI)== | ==Diagnosis of Recurrent Myocardial Infarction (re-MI)== | ||
| Line 230: | Line 230: | ||
In patients where recurrent myocardial infarction is suspected from clinical signs or symptoms following the initial infarction, an immediate measurement of the employed cardiac marker is recommended. A second sample should be obtained 3–6 h later. | In patients where recurrent myocardial infarction is suspected from clinical signs or symptoms following the initial infarction, an immediate measurement of the employed cardiac marker is recommended. A second sample should be obtained 3–6 h later. | ||
Recurrent myocardial infarction is diagnosed if there is a >20% increase of the value in the second sample. Although CK-MB used to be a test of choice for detecting recurrent myocardial infarction, trials data supports troponin use for the same purpose <ref>Apple FS, Murakami MM | Recurrent myocardial infarction is diagnosed if there is a >20% increase of the value in the second sample. Although CK-MB used to be a test of choice for detecting recurrent myocardial infarction, trials data supports troponin use for the same purpose <ref name="pmid15563477">{{cite journal |author=Apple FS, Murakami MM |title=Cardiac troponin and creatine kinase MB monitoring during in-hospital myocardial reinfarction |journal=Clin. Chem. |volume=51 |issue=2 |pages=460–3 |year=2005 |month=February |pmid=15563477 |doi=10.1373/clinchem.2004.042887 |url=}}</ref>. | ||
The re-elevation of the [[ST segment]] can, however, also be seen in threatening myocardial rupture and should lead to additional diagnostic work-up. Periodical and frequent [[EKG]] recordings, continuous monitoring of [[ST segment]]s with bedside monitors or holter systems might be helpful for early diagnosis. | The re-elevation of the [[ST segment]] can, however, also be seen in threatening myocardial rupture and should lead to additional diagnostic work-up. Periodical and frequent [[EKG]] recordings, continuous monitoring of [[ST segment]]s with bedside monitors or holter systems might be helpful for early diagnosis. | ||
| Line 238: | Line 238: | ||
==Prior Myocardial Infarction== | ==Prior Myocardial Infarction== | ||
The specificity of the [[EKG]] diagnosis for myocardial infarction is greatest when [[Q wave]]s occur in several leads or lead groups. [[ST segment]] deviations or [[T wave]]s alone are non-specific findings for myocardial necrosis. However, when these abnormalities occur in the same leads as the [[Q wave]]s, the likelihood of myocardial infarction is increased. For example, minor [[Q wave]]s ≥0.02 and <0.03 s that are ≥0.1 mV deep are suggestive of prior infarction if accompanied by inverted [[T wave]]s in the same lead group. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref>Savage RM, Wagner GS, Ideker RE, Podolsky SA, Hackel DB | The specificity of the [[EKG]] diagnosis for myocardial infarction is greatest when [[Q wave]]s occur in several leads or lead groups. [[ST segment]] deviations or [[T wave]]s alone are non-specific findings for myocardial necrosis. However, when these abnormalities occur in the same leads as the [[Q wave]]s, the likelihood of myocardial infarction is increased. For example, minor [[Q wave]]s ≥0.02 and <0.03 s that are ≥0.1 mV deep are suggestive of prior infarction if accompanied by inverted [[T wave]]s in the same lead group. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref><ref name="pmid832343">{{cite journal |author=Savage RM, Wagner GS, Ideker RE, Podolsky SA, Hackel DB |title=Correlation of postmortem anatomic findings with electrocardiographic changes in patients with myocardial infarction: retrospective study of patients with typical anterior and posterior infarcts |journal=Circulation |volume=55 |issue=2 |pages=279–85 |year=1977 |month=February |pmid=832343 |doi= |url=}}</ref><ref name="pmid5544988">{{cite journal |author=Horan LG, Flowers NC, Johnson JC |title=Significance of the diagnostic Q wave of myocardial infarction |journal=Circulation |volume=43 |issue=3 |pages=428–36 |year=1971 |month=March |pmid=5544988 |doi= |url=}}</ref><ref name=" Pahlm-1998">{{cite journal | author= Pahlm US, Chaitman BR, Rautaharju PM, Selvester RH, Wagner GS.| title= Comparison of the various electrocardiographic scoring codes for estimating anatomically documented sizes of single and multiple infarcts of the left ventricle.| journal= Am J Cardiol | year=1998 | volume=81 | pages=809-15| id= PMID 9555767}}</ref> | ||
Other validated myocardial infarction coding algorithms, such as the Minnesota code, Novacode, and WHO MONICA, define [[Q wave]] depth on the basis of depth, width, and ratio of R wave amplitude, such as [[Q wave]] depth at least one-third or one-fifth of R wave amplitude, and have been used extensively in epidemiological studies and clinical trials. <ref name=" Pahlm-1998">{{cite journal | author= Pahlm US, Chaitman BR, Rautaharju PM, Selvester RH, Wagner GS.| title= Comparison of the various electrocardiographic scoring codes for estimating anatomically documented sizes of single and multiple infarcts of the left ventricle.| journal= Am J Cardiol | year=1998 | volume=81 | pages=809-15| id= PMID 9555767}}</ref> <ref>Porela P, Helenius H, Pulkki K, Voipio-Pulkki LM | Other validated myocardial infarction coding algorithms, such as the Minnesota code, Novacode, and WHO MONICA, define [[Q wave]] depth on the basis of depth, width, and ratio of R wave amplitude, such as [[Q wave]] depth at least one-third or one-fifth of R wave amplitude, and have been used extensively in epidemiological studies and clinical trials. <ref name=" Pahlm-1998">{{cite journal | author= Pahlm US, Chaitman BR, Rautaharju PM, Selvester RH, Wagner GS.| title= Comparison of the various electrocardiographic scoring codes for estimating anatomically documented sizes of single and multiple infarcts of the left ventricle.| journal= Am J Cardiol | year=1998 | volume=81 | pages=809-15| id= PMID 9555767}}</ref><ref name="pmid10493844">{{cite journal |author=Porela P, Helenius H, Pulkki K, Voipio-Pulkki LM |title=Epidemiological classification of acute myocardial infarction: time for a change? |journal=Eur. Heart J. |volume=20 |issue=20 |pages=1459–64 |year=1999 |month=October |pmid=10493844 |doi=10.1053/euhj.1998.1529 |url=}}</ref><ref name="pmid9682893">{{cite journal |author=Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM |title=The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression |journal=J Electrocardiol |volume=31 |issue=3 |pages=157–87 |year=1998 |month=July |pmid=9682893 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S0022-0736(98)90132-7}}</ref> | ||
==Diagnosis of Myocardial Infarction Associated with Coronary Revascularization Procedures== | ==Diagnosis of Myocardial Infarction Associated with Coronary Revascularization Procedures== | ||
| Line 246: | Line 246: | ||
The [[EKG]] abnormalities that occur during or after percutaneous coronary intervention ([[PCI]]) are similar to those seen during spontaneous myocardial infarction. Injury can be due to embolization, vasoconstriction, edema, or reperfusion injury that occur as part of either [[PCI]] or [[CABG]]. Multiple events that can lead to myocardial necrosis are taking place, often in combination, during both types of intervention. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref name=" Ricciardi-2003">{{cite journal | author= Ricciardi MJ, Davidson CJ, Gubermikoff G, Beohar N, Eckman LJ, Parker MA, Bonow RO.| title= Troponin I elevation and cardiac events after percutaneous coronary intervention | journal= Am Heart J. | year=2003 | volume=145 | pages=522-28 | id= PMID 12660677}}</ref> <ref name=" Noora-2005">{{cite journal | author= Noora J, Ricci C, Hastings D, Hills S, Cybulsky I. | title= Determination of troponin I release after CABG surgery | journal= J Card Surg | year=1998 | volume=20 | pages=129-35| id= PMID 15725136}}</ref> | The [[EKG]] abnormalities that occur during or after percutaneous coronary intervention ([[PCI]]) are similar to those seen during spontaneous myocardial infarction. Injury can be due to embolization, vasoconstriction, edema, or reperfusion injury that occur as part of either [[PCI]] or [[CABG]]. Multiple events that can lead to myocardial necrosis are taking place, often in combination, during both types of intervention. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref name=" Ricciardi-2003">{{cite journal | author= Ricciardi MJ, Davidson CJ, Gubermikoff G, Beohar N, Eckman LJ, Parker MA, Bonow RO.| title= Troponin I elevation and cardiac events after percutaneous coronary intervention | journal= Am Heart J. | year=2003 | volume=145 | pages=522-28 | id= PMID 12660677}}</ref> <ref name=" Noora-2005">{{cite journal | author= Noora J, Ricci C, Hastings D, Hills S, Cybulsky I. | title= Determination of troponin I release after CABG surgery | journal= J Card Surg | year=1998 | volume=20 | pages=129-35| id= PMID 15725136}}</ref> | ||
In patients who have undergone [[CABG|CABG surgery]], new [[ST segment]] - [[T wave]] abnormalities are common but not necessarily diagnostic of myocardial ischemia.<ref>Yokoyama Y, Chaitman BR, Hardison RM, | In patients who have undergone [[CABG|CABG surgery]], new [[ST segment]] - [[T wave]] abnormalities are common but not necessarily diagnostic of myocardial ischemia.<ref name="pmid11024394">{{cite journal |author=Yokoyama Y, Chaitman BR, Hardison RM, ''et al'' |title=Association between new electrocardiographic abnormalities after coronary revascularization and five-year cardiac mortality in BARI randomized and registry patients |journal=Am. J. Cardiol. |volume=86 |issue=8 |pages=819–24 |year=2000 |month=October |pmid=11024394 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S0002-9149(00)01099-7}}</ref> | ||
When new pathological [[Q wave]]s appear in territories other than those identified before [[CABG|CABG surgery]], myocardial infarction should be considered, particularly if associated with elevated biomarkers, new wall motion abnormalities, or hemodynamic instability. | When new pathological [[Q wave]]s appear in territories other than those identified before [[CABG|CABG surgery]], myocardial infarction should be considered, particularly if associated with elevated biomarkers, new wall motion abnormalities, or hemodynamic instability. | ||
| Line 252: | Line 252: | ||
===Diagnosis of Myocardial Infarction in Patients Undergoing [[PCI|Percutaneous Coronary Intervention]]=== | ===Diagnosis of Myocardial Infarction in Patients Undergoing [[PCI|Percutaneous Coronary Intervention]]=== | ||
During [[PCI]], myocardial necrosis may result from recognizable peri-procedural events, alone or in combination, such as side-branch occlusion, disruption of collateral flow, distal embolization, coronary artery dissection, slow flow or no-reflow phenomenon, and microvascular plugging. Embolization of intracoronary thrombus or atherosclerotic particulate debris cannot be entirely prevented despite current antithrombotic and antiplatelet adjunctive therapy or protection devices. Such events induce extensive inflammation of non-infarcted myocardium surrounding small islets of myocardium necrosis. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref>Akkerhuis KM, Alexander JH, Tardiff BE, | During [[PCI]], myocardial necrosis may result from recognizable peri-procedural events, alone or in combination, such as side-branch occlusion, disruption of collateral flow, distal embolization, coronary artery dissection, slow flow or no-reflow phenomenon, and microvascular plugging. Embolization of intracoronary thrombus or atherosclerotic particulate debris cannot be entirely prevented despite current antithrombotic and antiplatelet adjunctive therapy or protection devices. Such events induce extensive inflammation of non-infarcted myocardium surrounding small islets of myocardium necrosis. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref name="pmid11827918">{{cite journal |author=Akkerhuis KM, Alexander JH, Tardiff BE, ''et al'' |title=Minor myocardial damage and prognosis: are spontaneous and percutaneous coronary intervention-related events different? |journal=Circulation |volume=105 |issue=5 |pages=554–6 |year=2002 |month=February |pmid=11827918 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=11827918}}</ref><ref name=" Herrman-2005">{{cite journal | author= Herrman J. | title= Peri-procedural myocardial injury: 2005 update | journal= Eur Heart J. | year=2005 | volume=26 | pages=2493–2519 | id= PMID 16176941}}</ref> <ref name=" Ricciardi-2003">{{cite journal | author= Ricciardi MJ, Davidson CJ, Gubermikoff G, Beohar N, Eckman LJ, Parker MA, Bonow RO.| title= Troponin I elevation and cardiac events after percutaneous coronary intervention | journal= Am Heart J. | year=2003 | volume=145 | pages=522-28 | id= PMID 12660677}}<ref name="pmid11387602">{{cite journal |author=Saadeddin SM, Habbab MA, Sobki SH, Ferns GA |title=Minor myocardial injury after elective uncomplicated successful PTCA with or without stenting: detection by cardiac troponins |journal=Catheter Cardiovasc Interv |volume=53 |issue=2 |pages=188–92 |year=2001 |month=June |pmid=11387602 |doi=10.1002/ccd.1146 |url=}}</ref><ref name="pmid11401931">{{cite journal |author=Ricciardi MJ, Wu E, Davidson CJ, ''et al'' |title=Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation |journal=Circulation |volume=103 |issue=23 |pages=2780–3 |year=2001 |month=June |pmid=11401931 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=11401931}}</ref> | ||
In the setting of [[PCI]], the balloon inflation during a procedure almost always results in cardiac ischemia whether or not accompanied by [[ST segment|ST]]-[[T wave|T]] changes. The occurrence of procedure-related cell necrosis can be detected by measurement of cardiac biomarkers before or immediately after the procedure, and again at 6–12 and 18–24 h.<ref>Gustavsson CG, Hansen O, Frennby B | In the setting of [[PCI]], the balloon inflation during a procedure almost always results in cardiac ischemia whether or not accompanied by [[ST segment|ST]]-[[T wave|T]] changes. The occurrence of procedure-related cell necrosis can be detected by measurement of cardiac biomarkers before or immediately after the procedure, and again at 6–12 and 18–24 h.<ref name="pmid15204231">{{cite journal |author=Gustavsson CG, Hansen O, Frennby B |title=Troponin must be measured before and after PCI to diagnose procedure-related myocardial injury |journal=Scand. Cardiovasc. J. |volume=38 |issue=2 |pages=75–9 |year=2004 |month=May |pmid=15204231 |doi=10.1080/14017430410026755 |url=}}</ref>{{cite journal | author= Miller WL, Garratt KN, Burrit MF, Lennon RJ, Reeder GS, Jaffe AS.| title= Baseline troponin level: key to understanding the importance of post-PCI troponin elevations. | journal= Eur Heart J. | year=2006 | volume=27 | pages=1061–69 | id= PMID 16481332}}</ref> Elevations of biomarkers above the 99th percentile URL after [[PCI]], assuming a normal baseline [[troponin]] value, are indicative of post-procedural myocardial necrosis. There is currently no solid scientific basis for defining a biomarker threshold for the diagnosis of peri-procedural myocardial infarction. Pending further data, and by arbitrary convention, it is suggested to designate increases more than three times the 99th percentile URL as [[PCI]] related myocardial infarction ('''type 4a'''). <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref name=" Herrman-2005">{{cite journal | author= Herrman J. | title= Peri-procedural myocardial injury: 2005 update | journal= Eur Heart J. | year=2005 | volume=26 | pages=2493–2519 | id= PMID 16176941}}</ref> | ||
If cardiac [[troponin]] is elevated before the procedure and not stable for at least two samples 6 h apart, there are insufficient | If cardiac [[troponin]] is elevated before the procedure and not stable for at least two samples 6 h apart, there are insufficient | ||
| Line 265: | Line 265: | ||
===Diagnosis of Myocardial Infarction in Patients Undergoing [[CABG|CABG surgery]]=== | ===Diagnosis of Myocardial Infarction in Patients Undergoing [[CABG|CABG surgery]]=== | ||
During [[CABG|CABG surgery]], numerous additional factors can lead to peri-procedural necrosis. These include direct myocardial trauma from sewing needles or manipulation of the heart, coronary dissection, global or regional ischemia related to inadequate cardiac protection, microvascular events related to reperfusion, myocardial damage induced by oxygen free radical generation, or failure to reperfuse areas of the myocardium that are not subtended by graftable vessels. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref name=" Kovacevic-2004">{{cite journal | author= Kovacevic R, Majkic-Singh N, Ignjatovic S, Otasevic P, Obrenovic R, Paris M, Vilotijevic B, Guermonprez JL.| title= Troponin T levels in detection of perioperative myocardial infarction after coronary artery bypass surgery | journal= Clin Lab.| year=2004 | volume=50 | pages=437-45 | id= PMID 15330513}}</ref> <ref name=" Noora-2005">{{cite journal | author= Noora J, Ricci C, Hastings D, Hills S, Cybulsky I. | title= Determination of troponin I release after CABG surgery | journal= J Card Surg | year=1998 | volume=20 | pages=129-35| id= PMID 15725136}}</ref> <ref>Benoit MO, Paris M, Silleran J, Fiemeyer A, Moatti N | During [[CABG|CABG surgery]], numerous additional factors can lead to peri-procedural necrosis. These include direct myocardial trauma from sewing needles or manipulation of the heart, coronary dissection, global or regional ischemia related to inadequate cardiac protection, microvascular events related to reperfusion, myocardial damage induced by oxygen free radical generation, or failure to reperfuse areas of the myocardium that are not subtended by graftable vessels. <ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> <ref name=" Kovacevic-2004">{{cite journal | author= Kovacevic R, Majkic-Singh N, Ignjatovic S, Otasevic P, Obrenovic R, Paris M, Vilotijevic B, Guermonprez JL.| title= Troponin T levels in detection of perioperative myocardial infarction after coronary artery bypass surgery | journal= Clin Lab.| year=2004 | volume=50 | pages=437-45 | id= PMID 15330513}}</ref> <ref name=" Noora-2005">{{cite journal | author= Noora J, Ricci C, Hastings D, Hills S, Cybulsky I. | title= Determination of troponin I release after CABG surgery | journal= J Card Surg | year=1998 | volume=20 | pages=129-35| id= PMID 15725136}}</ref> <ref name="pmid11588444">{{cite journal |author=Benoit MO, Paris M, Silleran J, Fiemeyer A, Moatti N |title=Cardiac troponin I: its contribution to the diagnosis of perioperative myocardial infarction and various complications of cardiac surgery |journal=Crit. Care Med. |volume=29 |issue=10 |pages=1880–6 |year=2001 |month=October |pmid=11588444 |doi= |url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0090-3493&volume=29&issue=10&spage=1880}}</ref> | ||
Any increase of cardiac biomarkers after [[CABG]] indicates myocyte necrosis, implying that an increasing magnitude of biomarker is likely to be related to an impaired outcome. This has been demonstrated in clinical studies employing CK-MB where elevations five, 10 and 20 times the upper limit of normal after [[CABG]] were associated with worsened prognosis. <ref>Costa MA, Carere RG, Lichtenstein SV, | Any increase of cardiac biomarkers after [[CABG]] indicates myocyte necrosis, implying that an increasing magnitude of biomarker is likely to be related to an impaired outcome. This has been demonstrated in clinical studies employing CK-MB where elevations five, 10 and 20 times the upper limit of normal after [[CABG]] were associated with worsened prognosis. <ref name="pmid11723020">{{cite journal |author=Costa MA, Carere RG, Lichtenstein SV, ''et al'' |title=Incidence, predictors, and significance of abnormal cardiac enzyme rise in patients treated with bypass surgery in the arterial revascularization therapies study (ARTS) |journal=Circulation |volume=104 |issue=22 |pages=2689–93 |year=2001 |month=November |pmid=11723020 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=11723020}}</ref><ref name="pmid11583884">{{cite journal |author=Klatte K, Chaitman BR, Theroux P, ''et al'' |title=Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial |journal=J. Am. Coll. Cardiol. |volume=38 |issue=4 |pages=1070–7 |year=2001 |month=October |pmid=11583884 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S0735-1097(01)01481-4}}</ref><ref name="pmid12475456">{{cite journal |author=Brener SJ, Lytle BW, Schneider JP, Ellis SG, Topol EJ |title=Association between CK-MB elevation after percutaneous or surgical revascularization and three-year mortality |journal=J. Am. Coll. Cardiol. |volume=40 |issue=11 |pages=1961–7 |year=2002 |month=December |pmid=12475456 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S073510970202538X}}</ref> | ||
Likewise, the increase of troponin levels after [[CABG]] indicates necrosis of myocardial cells, which predicts a poor outcome, in particular when elevated to the highest quartile or quintile of the troponin measurements.<ref>Januzzi JL, Lewandrowski K, MacGillivray TE, | Likewise, the increase of troponin levels after [[CABG]] indicates necrosis of myocardial cells, which predicts a poor outcome, in particular when elevated to the highest quartile or quintile of the troponin measurements.<ref name="pmid11985917">{{cite journal |author=Januzzi JL, Lewandrowski K, MacGillivray TE, ''et al'' |title=A comparison of cardiac troponin T and creatine kinase-MB for patient evaluation after cardiac surgery |journal=J. Am. Coll. Cardiol. |volume=39 |issue=9 |pages=1518–23 |year=2002 |month=May |pmid=11985917 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S0735109702017898}}</ref><ref name="pmid17000912">{{cite journal |author=Croal BL, Hillis GS, Gibson PH, ''et al'' |title=Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery |journal=Circulation |volume=114 |issue=14 |pages=1468–75 |year=2006 |month=October |pmid=17000912 |doi=10.1161/CIRCULATIONAHA.105.602370 |url=}}</ref> | ||
Unlike the prognosis, scant literature exists concerning the use of biomarkers for defining myocardial infarction in the setting of [[CABG]]. Therefore, '''biomarkers cannot stand alone in diagnosing myocardial infarction (type 5)'''.<ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> | Unlike the prognosis, scant literature exists concerning the use of biomarkers for defining myocardial infarction in the setting of [[CABG]]. Therefore, '''biomarkers cannot stand alone in diagnosing myocardial infarction (type 5)'''.<ref name=" Thygesen-2007">{{cite journal | author= Thygesen K, Alpert JS, White HD | title=Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF| journal=Circulation | year=2007 | volume=2007 | pages=2634–2653 | id=PMID 17951284}}</ref> | ||
Revision as of 16:31, 11 February 2009
| Myocardial infarction | |
| ICD-10 | I21-I22 |
|---|---|
| ICD-9 | 410 |
| DiseasesDB | 8664 |
| MedlinePlus | 000195 |
| eMedicine | med/1567 emerg/327 ped/2520 |
| Cardiology Network |
 Discuss Clinical classification of acute myocardial infarction further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Prior MI Classification Schemes
There have been several prior classification schemes for characterizing MI:
1. Transmural (necrosis of full thickness of ventricle) vs. non transmural (necrosis of partial thickness of ventricle)
2. Q wave vs. non Q wave: Based upon the development of electrocardiographic Q waves representing electrically inert tissue.
3. ST elevation MI (STEMI) and Non ST elevation myocardial infarction (NSTEMI)
At one time it was thought that Transmural MI and Q wave MI were synonymous. However, not all Q wave MIs are transmural, and not all transmural MIs are associated with Q waves.
Likewise, not all ST elevation MIs go on to cause q waves. Non ST elevation MIs can result in q waves.
Thus, ST elevation MI should not be equated with transmural MI or q wave MI. Likewise, Non ST elevation MI should not be equated with non transmural MI or non q wave MI. These 3 designations reflect three separate but overlapping characterization schemes.
New MI Clinical Classification System
A new clinical evidence based classification system has been introduced by Thygesen K, Alpert JS, White HD, et al. and jointly sponsored by the American College of Cardiology (ACC), American Heart Association (AHA), European Society of Cardiology (ESC), and the World Heart Federation (WHF).[1]
Definitions
Myocardial infarction can be characterized pathologically as acute, healing or healed.
Acute phase of myocardial infarction is characterized by the presence of polymorphonuclear leukocytes. If the time interval between the onset of the infarction and death is quite brief, e.g. 6 hours, minimal or no polymorphonuclear leukocytes may be seen.
The presence of mononuclear cells and fibroblasts, and the absence of polymorphonuclear leukocytes characterize healing infarction.
Healed infarction is characterized by scar tissue without cellular infiltration. The entire process leading to a healed infarction usually takes at least 5–6 weeks.
Reperfusion may alter the macroscopic and microscopic appearance of the necrotic zone by producing myocytes with contraction bands and large quantities of extravasated erythrocytes.
Myocardial infarctions can be classified temporally from clinical and other features, as well as according to the pathological appearance as:[1]
- Evolving phase of myocardial infarction: (>6 hours),
- Acute phase of myocardial infarction: (6 hours – 7 days),
- Healing phase of myocardial infarction: (7–28 days),
- Healed phase of myocardial infarction: (29 days and beyond).
It should be emphasized that the clinical and electrocardiographic timing of the onset of an acute myocardial infarction may not correspond precisely with the pathologic findings. For example, the EKG may still demonstrate evolving ST segment / T wave changes and cardiac biomarkers may still be elevated (implying a recent infarct) at a time when pathologically the infarction is in the healing phase. [2]
Pathology of Myocardial Infarction
Myocardial infarction is defined pathologically as myocardial cell death due to prolonged ischemia. Cell death is categorized pathologically as coagulation and/or contraction band necrosis, which usually evolves through oncosis (early primary necrosis), but can result to a lesser degree from apoptosis. Careful analysis of histological sections by an experienced observer is essential to distinguish these entities.[1] [2]
After the onset of myocardial ischemia, cell death is not immediate but takes a brief period of time to develop (as little as 20 minutes or less in some animal models). It takes several hours before myocardial necrosis can be identified by macroscopic or microscopic postmortem examination. [2]
Complete necrosis of all myocardial cells at risk requires at least 2–4 hours or longer depending on the presence of collateral circulation to the ischemic zone, whether the occlusion was persistent or intermittent, the sensitivity of the myocytes to ischemia, preconditioning, and/or, finally, individual demand for myocardial oxygen and nutrients. Myocardial infarctions are usually classified by the following:[1] [2]
A. Size:
- Microscopic: Focal necrosis,
- Small: <10% of the left ventricular myocardium,
- Moderate: 10–30% of the left ventricular myocardium,
- Large: >30% of the left ventricular myocardium.
B. Location: The pathological identification of the location of myocardial necrosis (anterior, posterior, lateral, inferior, apical) is made without reference to the coronary arterial tree
C. Clinical history.
Clinical Presentation of Ischemia
The term myocardial infarction indicates the presence of cell death of cardiac myocytes caused by ischemia, which is the result of a perfusion imbalance between myocardial oxygen supply and myocardial oxygen demand.
Ischemia in the clinical setting most often can be identified from the patient’s history and from the EKG. Possible ischemic symptoms include various combinations of chest, upper extremity, jaw, or epigastric discomfort with exertion or at rest.
The discomfort associated with acute myocardial infarction usually lasts at least 20 minutes. Often, the discomfort is diffuse, not localized, not positional, not affected by movement of the region, and it may be accompanied by dyspnea, diaphoresis, nausea, or syncope.
These symptoms are not specific to myocardial ischemia and can be misdiagnosed and thus attributed to gastrointestinal, neurological, pulmonary, or musculoskeletal disorders.
Myocardial infarction may occur with atypical symptoms, or even without symptoms, being detected only by EKG, elevation of biomarkers, or cardiac imaging.[1]
Criteria for Diagnosis of Acute Myocardial Infarction
The term myocardial infarction should be used when there is evidence of myocardial necrosis in a clinical setting consistent with myocardial ischemia. Under these conditions any one of the following criteria meets the diagnosis for acute myocardial infarction. [1]
- Detection of rise and/or fall of cardiac biomarkers (preferably Troponin) with at least one of the following
- Sudden unexpected cardiac death, including cardiac arrest, often with symptoms suggestive of myocardial ischemia, accompanied by presumably new ST segment elevation, or new LBBB, and/or evidence of fresh thrombus in a coronary artery by angiography and/or at autopsy, if death has occurred before blood samples could be obtained, or at a time before the appearance of cardiac biomarkers in the blood
- In patients with normal baseline troponin values, a greater than 3 times increase above the 99th percentile of the upper limit of normal of cardiac biomarkers has been designated as the definition of PCI related myocardial infarction. A subtype related to documented stent thrombosis is recognized.
- For patients with CABG surgery; (In patients with normal baseline troponin values) increases of cardiac biomarkers greater than 5 times, (> 5 times the 99th percentile upper limit of normal) and either new pathological Q waves or new LBBB or angiographically evidence of new graft or native vessel occlusion have been designated as defining CABG surgery related myocardial infarction.
- Pathological findings of acute myocardial infarction.
Criteria for Prior Myocardial Infarction
Any of the following criteria meets the diagnosis for prior myocardial infarction:[1]
- Development of new pathological Q waves with or without symptoms
- Imaging evidence of a region of loss of viable myocardium that is thinned and fails to contract in the absence of a non ischemic cause.
- Pathological findings of healed or healing myocardial infarction.
Classification
Clinically the various types of myocardial infarction can be classified as follow: [1]
- Spontaneous myocardial infarction related to ischemia due to a primary coronary event, such as plaque erosion and/or rupture, fissuring, or dissection.
- Myocardial infarction secondary to ischemia due to an imbalance of O2 supply and demand, as from coronary spasm or embolism, anemia, arrhythmias, hypertension, or hypotension
- Sudden unexpected cardiac death, including cardiac arrest, often with symptoms suggesting ischemia with new ST segment elevation; new left bundle branch block; or pathologic or angiographic evidence of fresh coronary thrombus (in the absence of reliable biomarker findings)
-
- a. Myocardial infarction associated with Percutaneous Coronary Interventions (PCI)
- b. Myocardial infarction associated with documented in stent thrombosis.
- Myocardial infarction associated with Coronary Artery Bypass Graft surgery
Diagnostic Applications for Acute Myocardial Infarction
Differential Diagnosis for EKG in Acute Myocardial Infarction
Conditions that confound the EKG diagnosis of myocardial infarction are the following: [1];
- A QS complex in lead V1 is normal.
- A Q wave <0.03 s and <1/4 of the R wave amplitude in lead III is normal if the frontal QRS axis is between 30 and 0°.
- The Q wave may be normal in aVL if the frontal QRS axis is between 60 and 90°. Small septal Q waves are non pathological Q waves if <0.03 s and <1/4 of the R wave amplitude in leads I, aVL, aVF, and V4-V5-V6
- The following may be associated with Q/QS complexes in the absence of myocardial infarction:
- Preexcitation syndromes
- Obstructive or dilated cardiomyopathy
- LBBB
- RBBB
- Left anterior fascicular block
- LVH
- RVH
- Myocarditis
- Acute cor pulmonale
- Hyperkalemia
Diseases That May be Confused with Acute MI
- Benign early repolarization (e.g. high take-off)
- Pericarditis
- LBBB
- Pulmonary embolism
- Myocarditis
- Brugada syndrome
- Preexcitation syndromes
- Subarachnoid hemorrhage
- Cholecystitis
- Electrolyte imbalance (hyperkalemia)
- Lead misplacements
- Different lead configurations (e.g. modified Mason-Likar lead configurations)
- Misevaluation of J point variations
Disease States that May Cause a False Negative Evaluation of the EKG in Acute MI
- Prior myocardial infarction with Q waves and/or persistent ST segment elevation.
- LBBB
- Paced rhythm
EKG Manifestations of Acute Myocardial Injury or Ischemia
EKG manifestations of acute myocardial injury or ischemia in absence of left ventricular hypertrophy and LBBB as follow[3][4]
- ST segment elevation
In general, ST segment elevation reflects myocardial injury, which may be irreversible (unlike ischemia which may be reversible) and which is associated with a risk of necrosis. ST elevation is defined as new ST segment elevation at the J point in two contiguous leads with the cut off points ≥0.2 mV in men or ≥0.15 mV in women in V2-V3 and ≥0.1 mV in other leads.
- ST segment depression and T wave changes
In general, ST segment depression represents reversible ischemia (less likely to result in irreversible necorsis). One exception is the presence of ST segment depression in the anterior precordial leads that can reflect posterior injury rather than anterior ischemia. Ischemia is defined as new horizontal or downsloping ST segment changes as ≥0.05 mV in two contiguous leads and/or T wave inversion ≥0.1 mV in two contiguous leads with prominent R wave or in situations which R wave amplitude / S wave amplitude ratio is >1.
Althought it is not observed in women, the J point elevation in men decreases with increasing age.[5]
The term of contiguous lead represents lead groups such as anterior leads (V1-V6), inferior leads (II, III, and aVF), or lateral/apical leads (I and aVL).
EKG Changes of Prior Myocardial Infarction
- Any Q wave in V2-V3 ≥0.02 sec or presence of QS complex in V2 and V3.
- Q wave ≥0.03 sec and ≥0.1 mV deep or presence of QS complexes in leads I, II, aVL, aVF or V4-V5-V6 in any two leads of a contiguous lead grouping (I, aVL, V6; V4-V5-V6, II, III and aVF). The same criteria are used for supplemental leads V7-V8-V9, and for the Cabrera frontal plane leads.
- R wave ≥0.04 sec in V1-V2 and R/S >1 with a concordant positive T wave in the absence of a conduction defect.[1]
Evaluation of Biomarkers
Myocardial cell death can be recognized by the appearance in the blood of different proteins released into the circulation from the damaged myocytes: myoglobin, cardiac troponin T (cTnT) and I (cTnI), CK (Creatine Kinase), LDH (Lactate Dehydrogenase), as well as many other enzyme markers of necrosis.[6]
Myocardial infarction is diagnosed when blood levels of sensitive and specific biomarkers such as cardiac troponins (T and I) or CK-MB are increased in the clinical setting of acute myocardial ischemia. [1]
Although elevations in these biomarkers reflect myocardial necrosis, they do not indicate its mechanism. Thus, an elevated value of cardiac troponin in the absence of clinical evidence of ischemia should prompt a search for other etiologies of myocardial necrosis. [1][7][8][9][10][11]
Electrocardiographic Detection of Myocardial Infarction
The acute or evolving changes in the ST segment, T wave and Q wave when present, potentially allow the clinician to date the event, to gain insight into the location of the infarct related artery, and to estimate the amount of myocardium at risk.
Coronary artery dominance, size and distribution of arterial segments, collateral vessels, and location, extent, and severity of coronary stenoses can also impact EKG manifestations of myocardial ischemia. The EKG is notoriously inaccurate in localizing which artery is the culprit.
The EKG by itself is often insufficient to diagnose acute myocardial ischemia or infarction since ST segment changes may be observed in other conditions. Also Q waves may occur due to myocardial fibrosis in the absence of coronary artery disease such as Chagas disease.[1][12][13]
Also, the EKG is a useful clinical marker of tissue perfusion. Greater ST segment resolution on the static and the continuous EKG correlate with TIMI grade 3 flow, TIMI Myocardial Perfusion Grade 3, smaller infarct sizes, and improved survival.[14]
Echocardiography
Echocardiography is a reliable real time imaging technique with moderate spatial and temporal resolution. Its strength is the assessment of myocardial thickness, thickening, and motion at rest. This can be aided by tissue Doppler imaging. [1][15]
Radionuclide Imaging
Several radionuclide tracers allow viable myocytes to be imaged directly, including thallium-201, technetium-99m MIBI, tetrofosmin, and [18F]2-fluorodeoxyglucose (FDG).[16][17][18]
Radionuclide assessment of perfusion at the time of patient presentation can be performed with immediate tracer injection and imaging that can be delayed for up to several hours. The technique is interpreter dependent, although objective quantitative analysis is available. EKG gating provides simultaneous information and reliable assessment on myocardial motion, thickening, and global left ventricular function. [1][19]
Multi Slice Computed Tomography
Infarcted myocardium is initially visible to computerized tomography as a focal area of decreased left ventricular enhancement, but later examination shows hyperenhancement as with late gadolinium imaging by MRI. Contrast enhanced CT may be performed and helpful for suspected embolism and aortic dissection, conditions with clinical features that overlap with those of acute myocardial infarction. [1] [20]
Cardiac Magnetic Resonance Imaging
Cardiac MRI (C-MRI) has high spatial resolution and moderate temporal resolution. Although not widely available and not commonly used in acute setting, it is a well validated standard for the assessment of myocardial function and has, in theory, similar capability to echocardiography in suspected acute myocardial infarction and can play an important role in its detection.
Paramagnetic contrast agents can be used to assess myocardial perfusion and the increase in extracellular space associated with the fibrosis of chronic phase of myocardial infarction.[1][21][22]
Diagnosis of Recurrent Myocardial Infarction (re-MI)
The EKG diagnosis of recurrent myocardial infarction (re-MI, reinfarction) following the initial infarction may be confounded by the initial evolutionary EKG changes.
Reinfarction should be considered when ST segment elevation ≥0.1 mV reoccurs in a patient having a lesser degree of ST segment elevation or new pathognomonic Q waves, in at least two contiguous leads, particularly when associated with ischemic symptoms for 20 min or longer.
In patients where recurrent myocardial infarction is suspected from clinical signs or symptoms following the initial infarction, an immediate measurement of the employed cardiac marker is recommended. A second sample should be obtained 3–6 h later.
Recurrent myocardial infarction is diagnosed if there is a >20% increase of the value in the second sample. Although CK-MB used to be a test of choice for detecting recurrent myocardial infarction, trials data supports troponin use for the same purpose [23].
The re-elevation of the ST segment can, however, also be seen in threatening myocardial rupture and should lead to additional diagnostic work-up. Periodical and frequent EKG recordings, continuous monitoring of ST segments with bedside monitors or holter systems might be helpful for early diagnosis.
ST segment depression or LBBB on their own should not be considered valid criteria for myocardial infarction.[1]
Prior Myocardial Infarction
The specificity of the EKG diagnosis for myocardial infarction is greatest when Q waves occur in several leads or lead groups. ST segment deviations or T waves alone are non-specific findings for myocardial necrosis. However, when these abnormalities occur in the same leads as the Q waves, the likelihood of myocardial infarction is increased. For example, minor Q waves ≥0.02 and <0.03 s that are ≥0.1 mV deep are suggestive of prior infarction if accompanied by inverted T waves in the same lead group. [1][24][25][26]
Other validated myocardial infarction coding algorithms, such as the Minnesota code, Novacode, and WHO MONICA, define Q wave depth on the basis of depth, width, and ratio of R wave amplitude, such as Q wave depth at least one-third or one-fifth of R wave amplitude, and have been used extensively in epidemiological studies and clinical trials. [26][27][28]
Diagnosis of Myocardial Infarction Associated with Coronary Revascularization Procedures
The EKG abnormalities that occur during or after percutaneous coronary intervention (PCI) are similar to those seen during spontaneous myocardial infarction. Injury can be due to embolization, vasoconstriction, edema, or reperfusion injury that occur as part of either PCI or CABG. Multiple events that can lead to myocardial necrosis are taking place, often in combination, during both types of intervention. [1] [29] [30]
In patients who have undergone CABG surgery, new ST segment - T wave abnormalities are common but not necessarily diagnostic of myocardial ischemia.[31]
When new pathological Q waves appear in territories other than those identified before CABG surgery, myocardial infarction should be considered, particularly if associated with elevated biomarkers, new wall motion abnormalities, or hemodynamic instability.
Diagnosis of Myocardial Infarction in Patients Undergoing Percutaneous Coronary Intervention
During PCI, myocardial necrosis may result from recognizable peri-procedural events, alone or in combination, such as side-branch occlusion, disruption of collateral flow, distal embolization, coronary artery dissection, slow flow or no-reflow phenomenon, and microvascular plugging. Embolization of intracoronary thrombus or atherosclerotic particulate debris cannot be entirely prevented despite current antithrombotic and antiplatelet adjunctive therapy or protection devices. Such events induce extensive inflammation of non-infarcted myocardium surrounding small islets of myocardium necrosis. [1] [32][33] [34]
In the setting of PCI, the balloon inflation during a procedure almost always results in cardiac ischemia whether or not accompanied by ST-T changes. The occurrence of procedure-related cell necrosis can be detected by measurement of cardiac biomarkers before or immediately after the procedure, and again at 6–12 and 18–24 h.[35]Miller WL, Garratt KN, Burrit MF, Lennon RJ, Reeder GS, Jaffe AS. (2006). "Baseline troponin level: key to understanding the importance of post-PCI troponin elevations". Eur Heart J. 27: 1061–69. PMID 16481332.</ref> Elevations of biomarkers above the 99th percentile URL after PCI, assuming a normal baseline troponin value, are indicative of post-procedural myocardial necrosis. There is currently no solid scientific basis for defining a biomarker threshold for the diagnosis of peri-procedural myocardial infarction. Pending further data, and by arbitrary convention, it is suggested to designate increases more than three times the 99th percentile URL as PCI related myocardial infarction (type 4a). [1] [33]
If cardiac troponin is elevated before the procedure and not stable for at least two samples 6 h apart, there are insufficient data to recommend biomarker criteria for the diagnosis of peri-procedural myocardial infarction.[36]
If the values are stable or falling, criteria for reinfarction by further measurement of biomarkers together with the features of the EKG or imaging can be applied.
A separate subcategory of myocardial infarction (type 4b) is related to stent thrombosis as documented by angiography and/or autopsy. Although iatrogenic, myocardial infarction type 4b with verified stent thrombosis must meet the criteria for spontaneous myocardial infarction as well.[1] [33]
Diagnosis of Myocardial Infarction in Patients Undergoing CABG surgery
During CABG surgery, numerous additional factors can lead to peri-procedural necrosis. These include direct myocardial trauma from sewing needles or manipulation of the heart, coronary dissection, global or regional ischemia related to inadequate cardiac protection, microvascular events related to reperfusion, myocardial damage induced by oxygen free radical generation, or failure to reperfuse areas of the myocardium that are not subtended by graftable vessels. [1] [37] [30] [38]
Any increase of cardiac biomarkers after CABG indicates myocyte necrosis, implying that an increasing magnitude of biomarker is likely to be related to an impaired outcome. This has been demonstrated in clinical studies employing CK-MB where elevations five, 10 and 20 times the upper limit of normal after CABG were associated with worsened prognosis. [39][40][41]
Likewise, the increase of troponin levels after CABG indicates necrosis of myocardial cells, which predicts a poor outcome, in particular when elevated to the highest quartile or quintile of the troponin measurements.[42][43]
Unlike the prognosis, scant literature exists concerning the use of biomarkers for defining myocardial infarction in the setting of CABG. Therefore, biomarkers cannot stand alone in diagnosing myocardial infarction (type 5).[1]
In view of the adverse impact on survival observed in patients with significant biomarker elevations, the most recent ACC/AHA/ESC/WHF Task Force suggests, by arbitrary convention, that biomarker values more than five times the 99th percentile of the normal reference range during the first 72 h following CABG, when associated with the appearance of new pathological Q waves or new LBBB, or angiographically documented new graft or native coronary artery occlusion, or imaging evidence of new loss of viable myocardium, should be considered as diagnostic of a CABG related myocardial infarction (type 5 myocardial infarction).[1] [37]
Application in the Setting of Myocardial Infarction
A. In acute phase
Imaging techniques can be useful in the diagnosis of myocardial infarction because of the ability to detect wall motion abnormalities in the presence of elevated cardiac biomarkers. An important role of acute echocardiography (in Emergency Department for triage or bedside]] or radionuclide imaging is in patients with suspected myocardial infarction and/or presence of a non diagnostic EKG. [1] [44] [45] [46] [47]
A normal echocardiogram or resting EKG gated scintigram has a 95–98% negative predictive value for excluding acute infarction.[1]
B. In the healing or healed phase
Imaging techniques are useful in myocardial infarction for analysis of left ventricular function, both at rest and during dynamic exercise or pharmacological stress, to provide an assessment of remote inducible ischemia. Non-invasive imaging techniques can diagnose healing or healed infarction by demonstrating regional wall motion, thinning, or scar in the absence of other causes.
Echocardiography and radionuclide techniques, in conjunction with exercise or pharmacological stress, can identify ischemia and myocardial viability.
The high resolution of contrast enhanced MRI (CE MRI) means that areas of late enhancement correlate well with areas of fibrosis and thereby enable differentiation between transmural and subendocardial scarring. CE MRI is potentially valuable in assessing left ventricular function and areas of viable and potentially hibernating myocardium.[1] [48] [49]
Impact on Clinical Trials in Which Myocardial Infarction is an Endpoint
In the design of a new study, clinical trial investigators should specify which definition of myocardial infarction they expect will be altered by the new treatment under investigation. Factors that should be considered include:[1] [50]
- Assessment of the incidence of spontaneous myocardial infarction (type 1) and infarction related to myocardial oxygen supplies and demand (type 2) in treated patients vs. control subjects.
- Assessment of the incidence of sudden death related to myocardial infarction when applying the suggested criteria (type 3).
- Assessment of the incidence of revascularization procedure related myocardial infarctions and biomarker elevations (for PCI, type 4a and type 4b; and for CABG, type 5).
Public Policy Implications of Redefinition of Myocardial Infarction
Increasing the sensitivity of diagnostic criteria for myocardial infarction may result in more cases being identified and identify more patients who would be appropriate for secondary prevention measures.
Increasing the specificity of diagnostic criteria for myocardial infarction will result in more accurate diagnosis but will not exclude the presence of coronary artery disease, patients who might benefit from secondary prevention. [1] [51]
In order to meet this challenge;
- Physicians must be adequately informed of the altered diagnostic criteria.
- Educational materials will need to be created, treatment guidelines must be appropriately adapted and freely delivered.
- Professional societies should take steps to facilitate the rapid dissemination of the revised definition to:
- a. Physicians,
- b. Other health care professionals,
- c. Administrators,
- d. The general public,
- e. Insurers,
- f. Regulatory authorities
Global Impact of Redefinition of Myocardial Infarction
Beyond the substantial impact on the identification, prevention, and treatment of cardiovascular disease throughout the world, the changes in the definition of acute myocardial infarction have critical consequences for less developed and developing countries. In many countries, the resources to apply the new definition may not be available in all hospitals. However, many developing countries already do have medical facilities capable of or currently employing the proposed definition of acute myocardial infarction. The new definition will also impact epidemiological data from developing countries related to prevalence and incidence of acute MI.[1]
In less advantaged hospitals, the diagnosis of myocardial infarction may depend mostly on clinical signs and symptoms coupled with less sophisticated biomarker analyses. Some of these institutions may only have access to Creatin Kinase and its isoenzymes at the present time.
The redefinition arises from and is compatible with the latest scientific knowledge and with advances in technology, particularly with regard to the use of biomarkers, high quality electrocardiography, and imaging techniques.
The definition can and should be used by developed countries immediately, and by developing countries as quickly as resources become available.[1]
Histopathological Examples
Images displayed in this section are courtesy of Professor Peter Anderson, and published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology PEIR
-
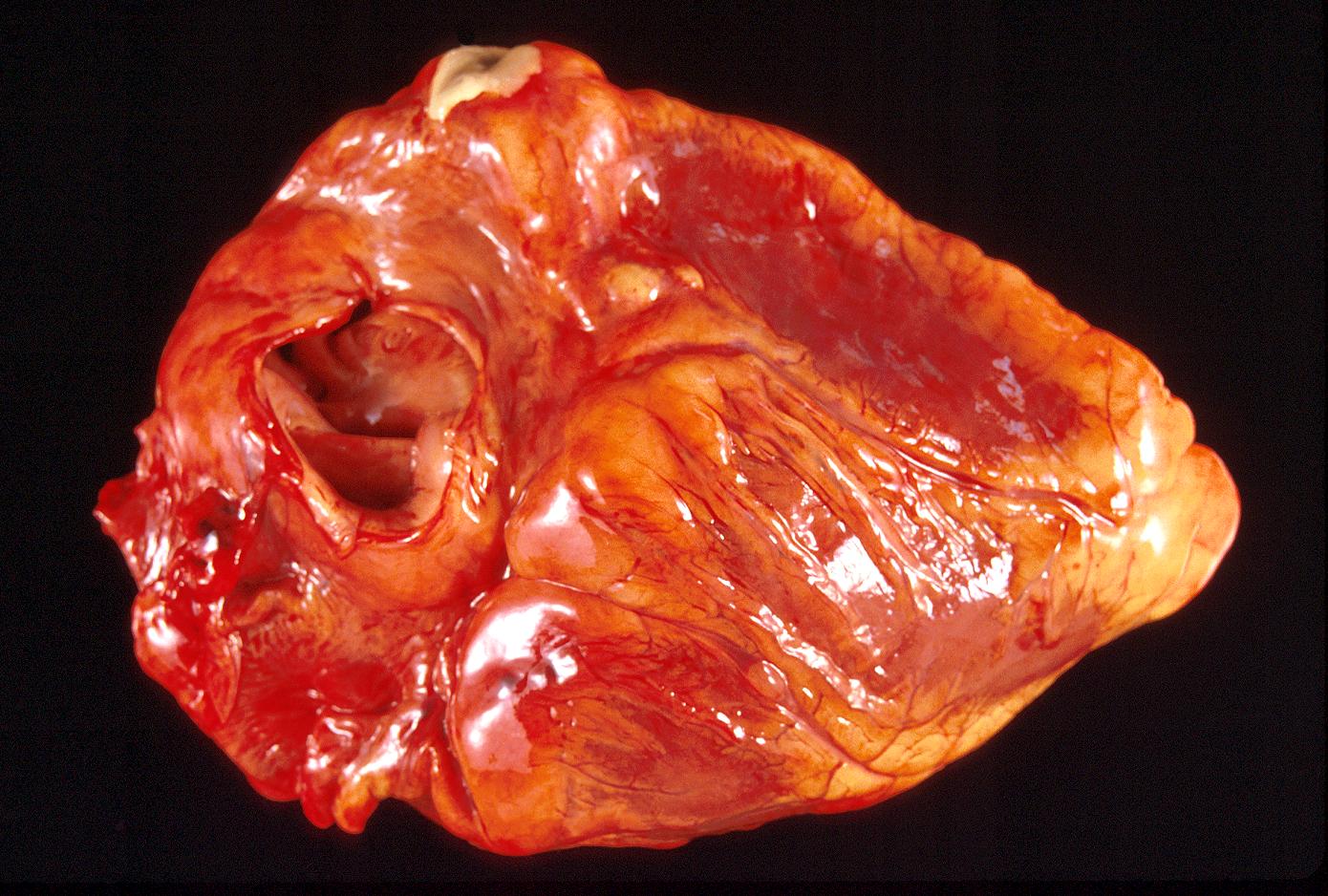
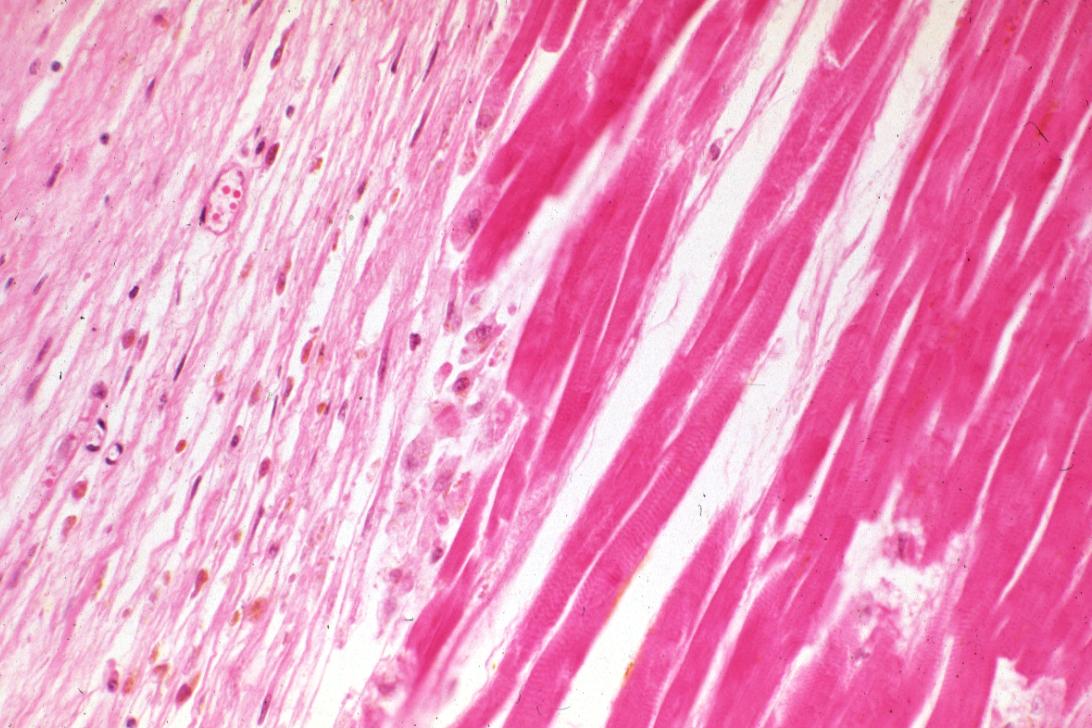
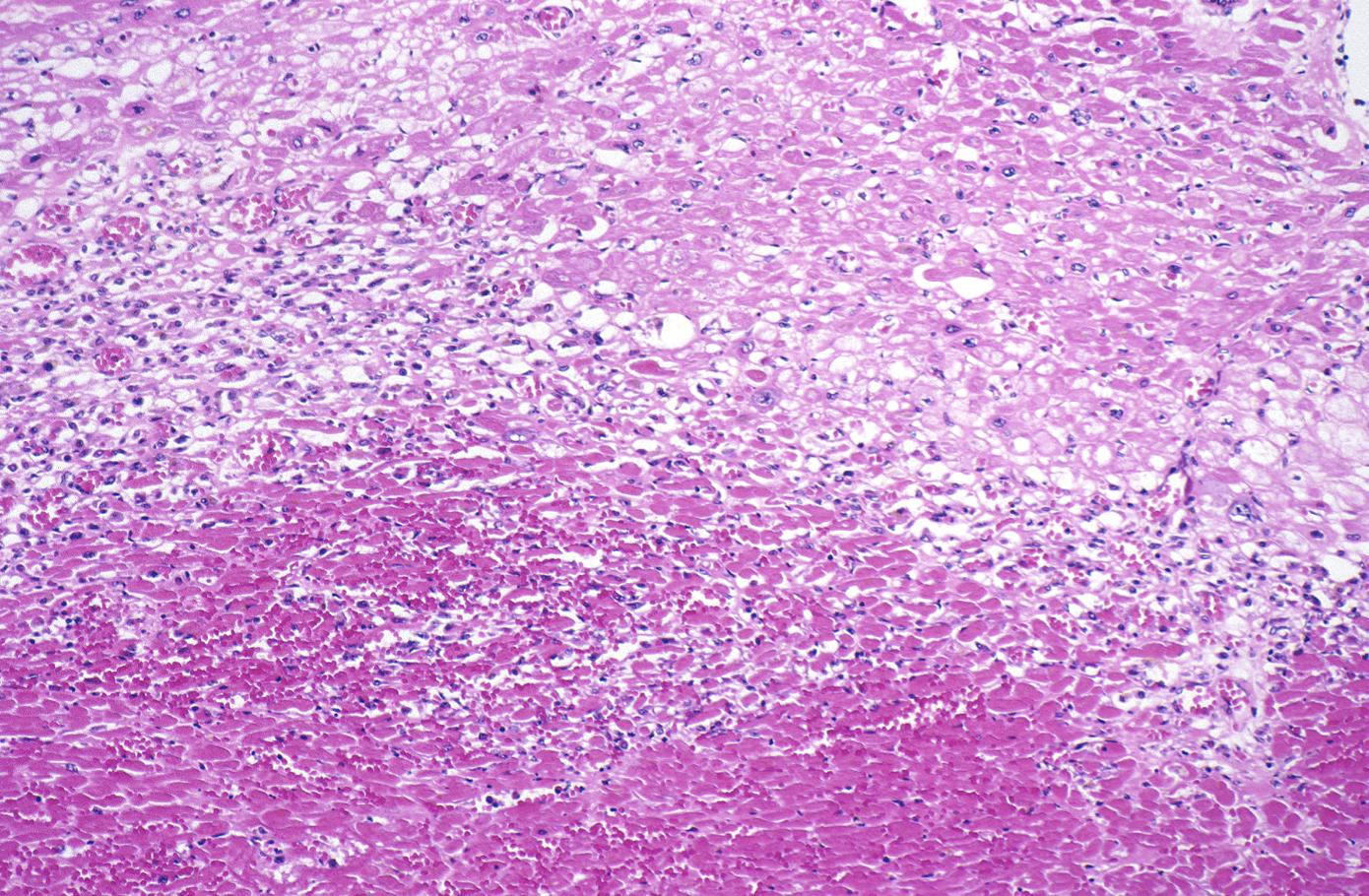
Acute Myocardial infarction, coagulative necrosis
-
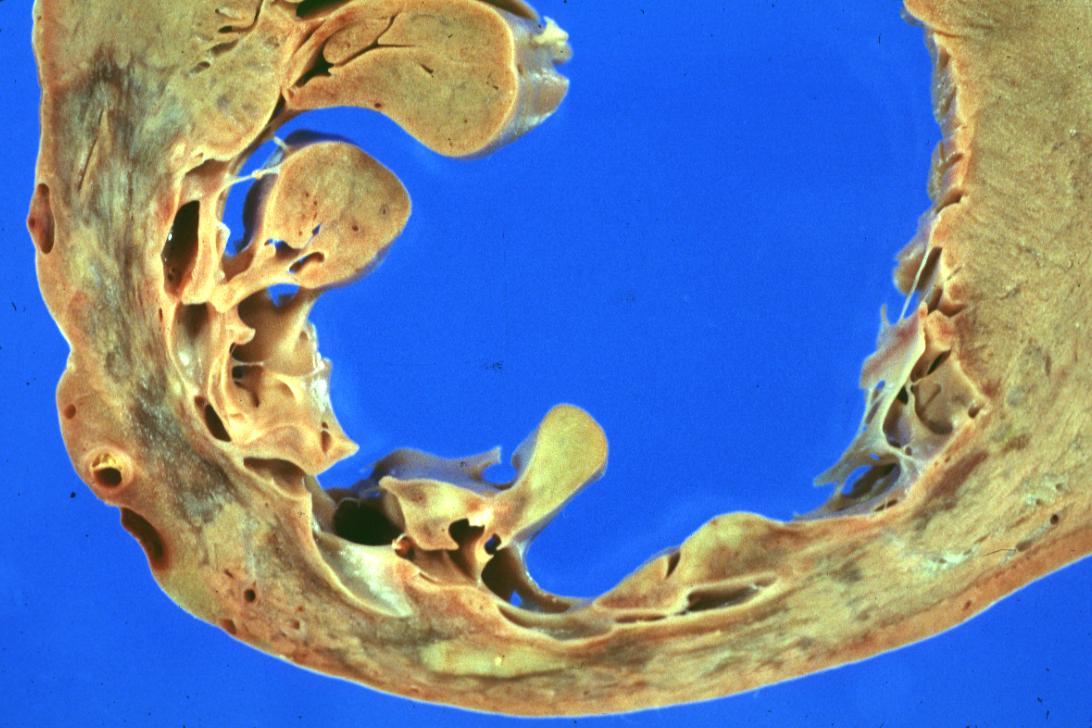
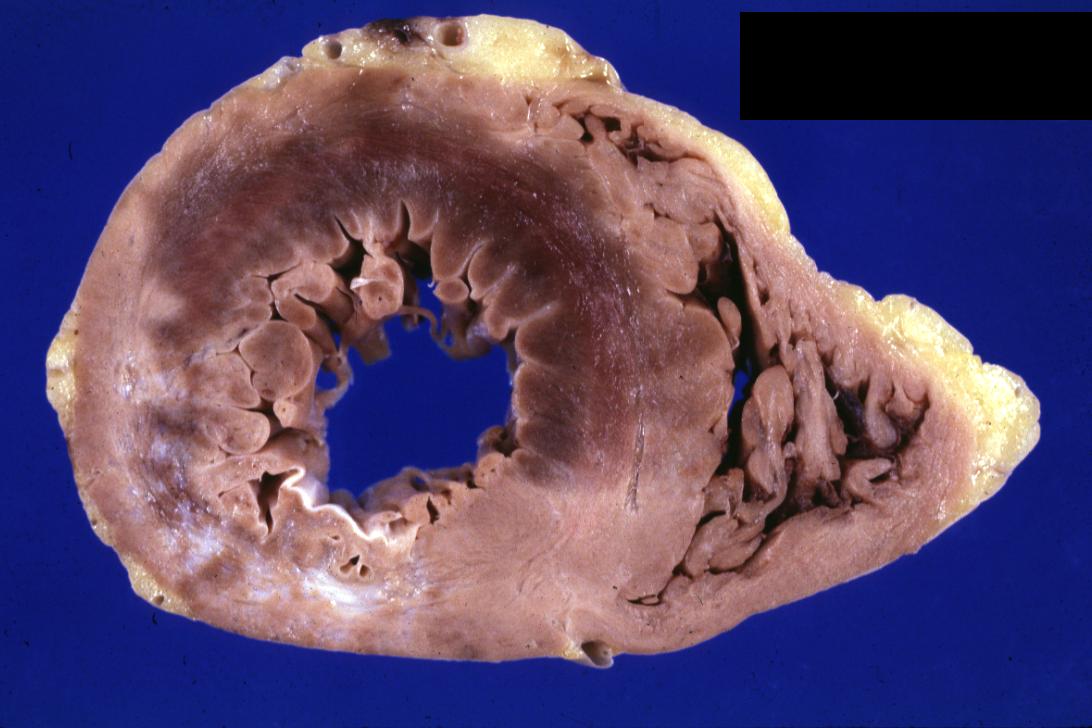
Gross example of myocardial infarction that is several weeks or perhaps months of age
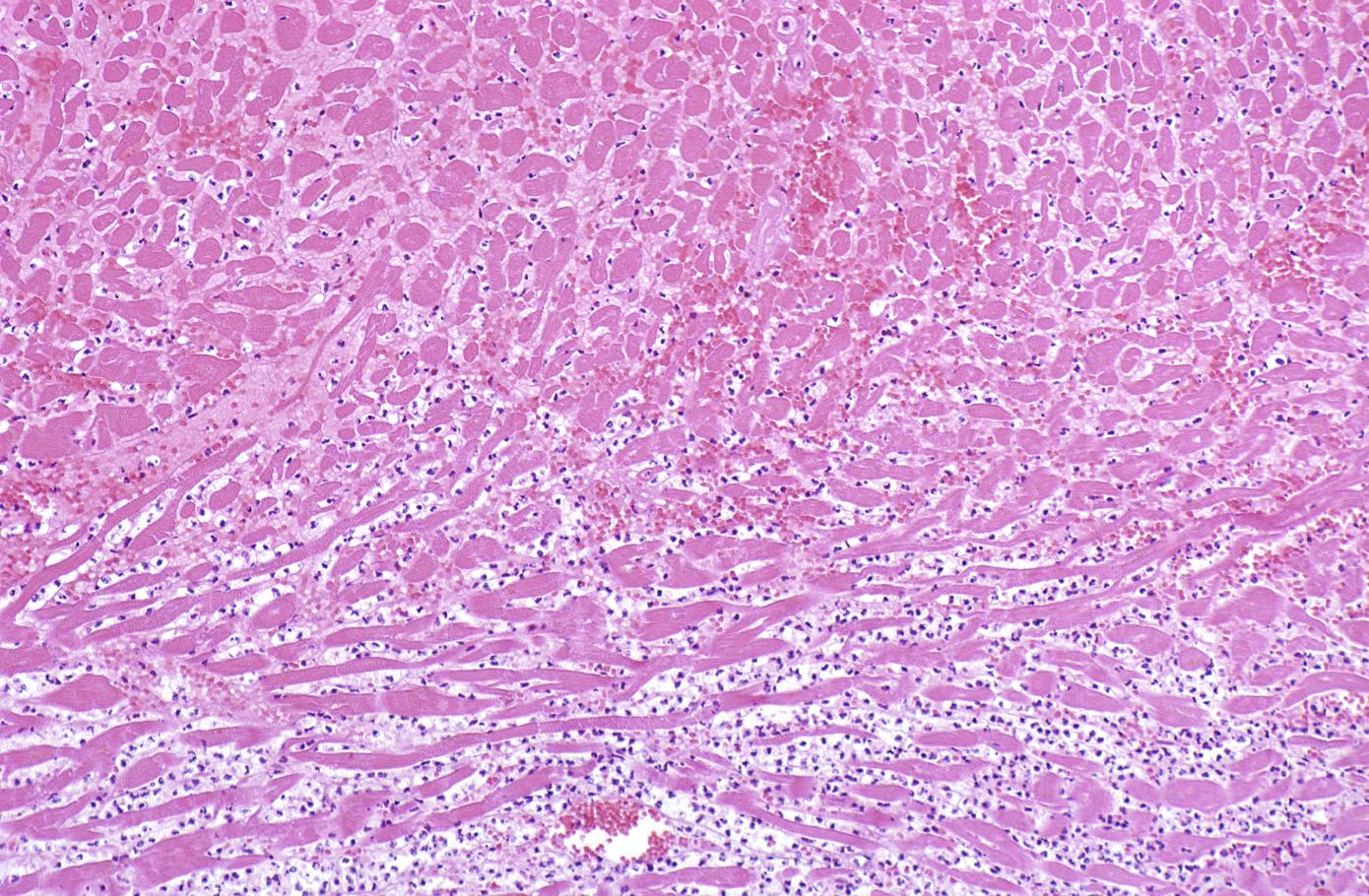
-
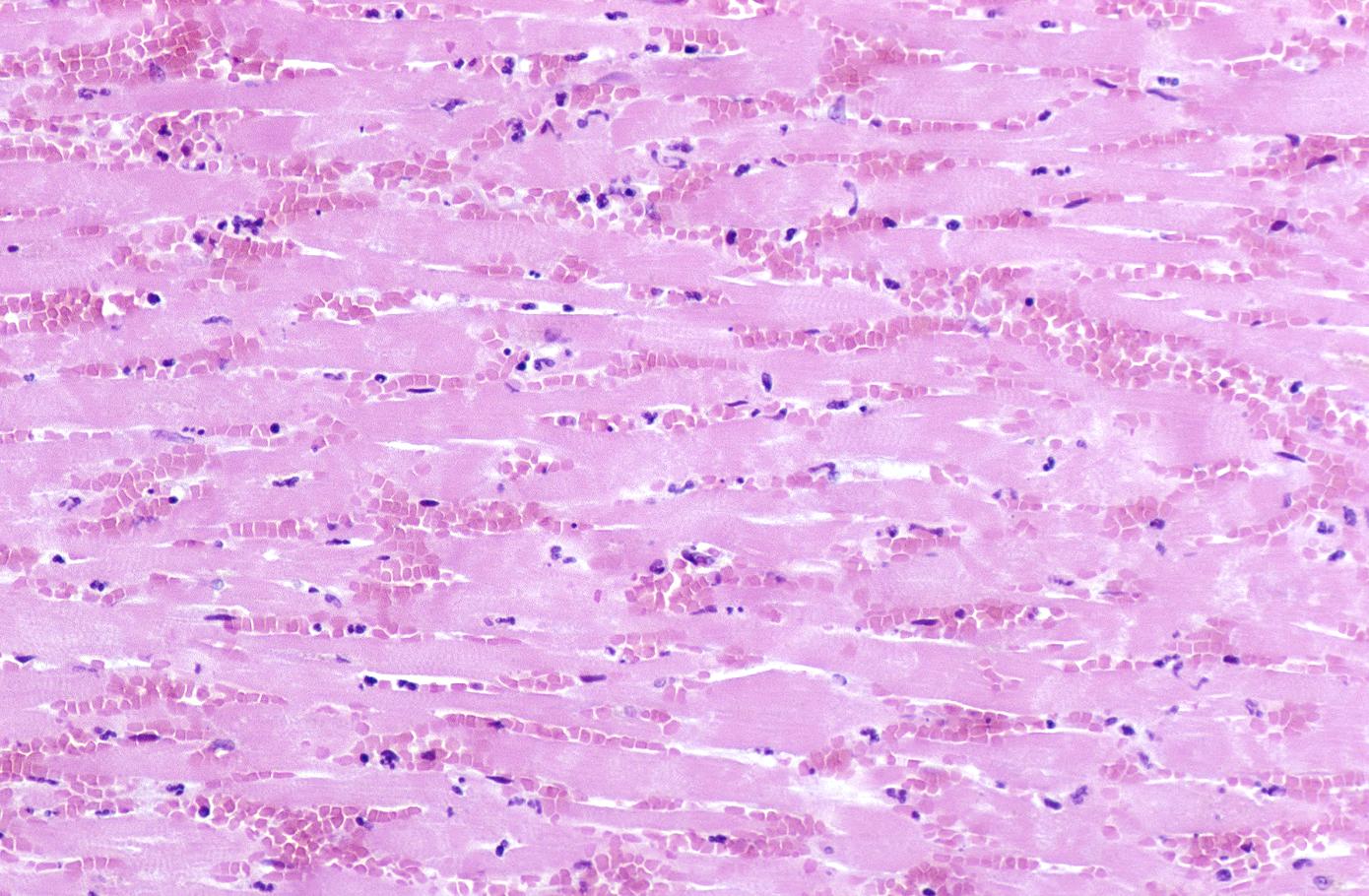
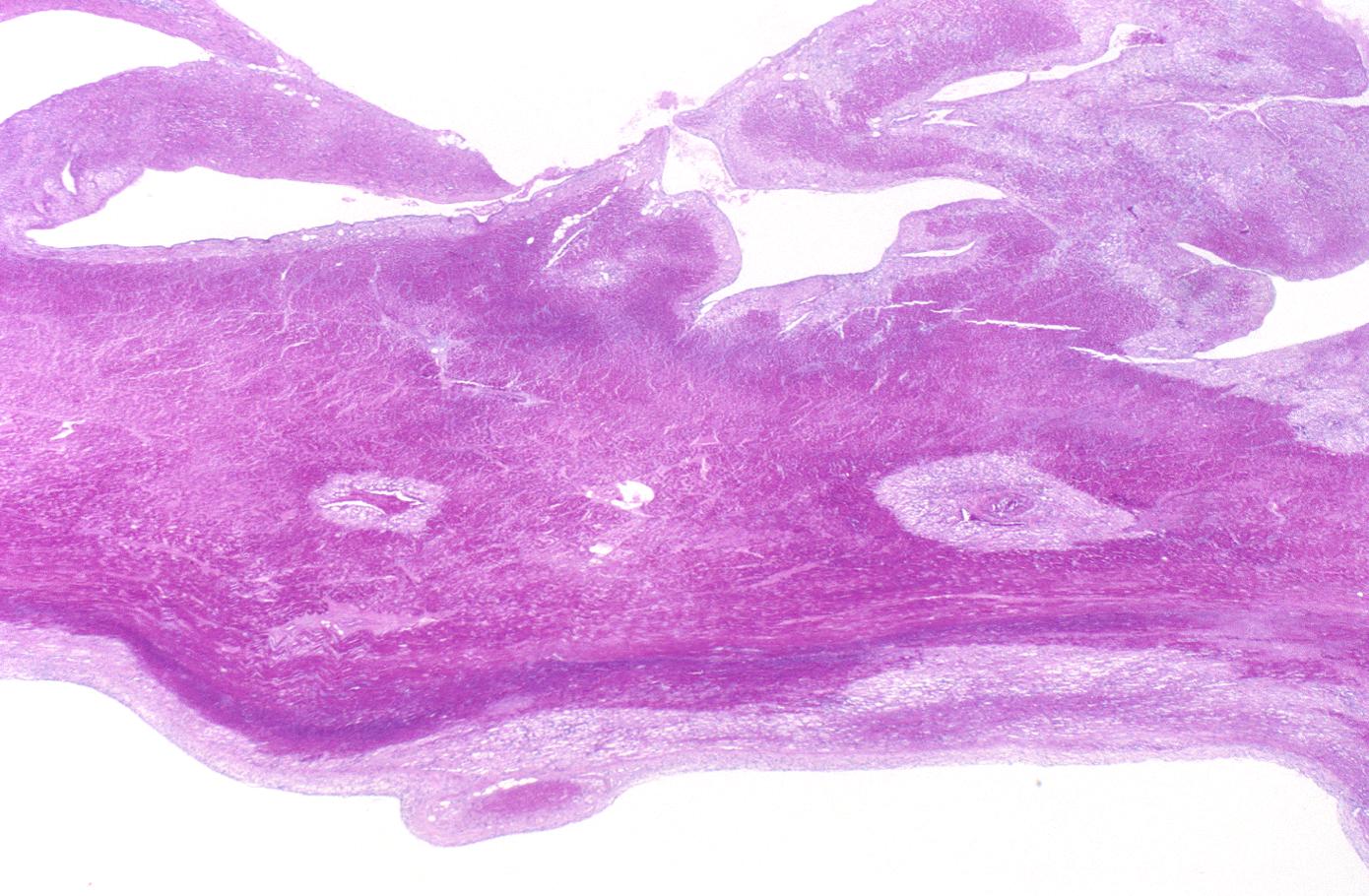
The same heart. Microscopic view. H&E, medium magnification view of healing infarct.
-
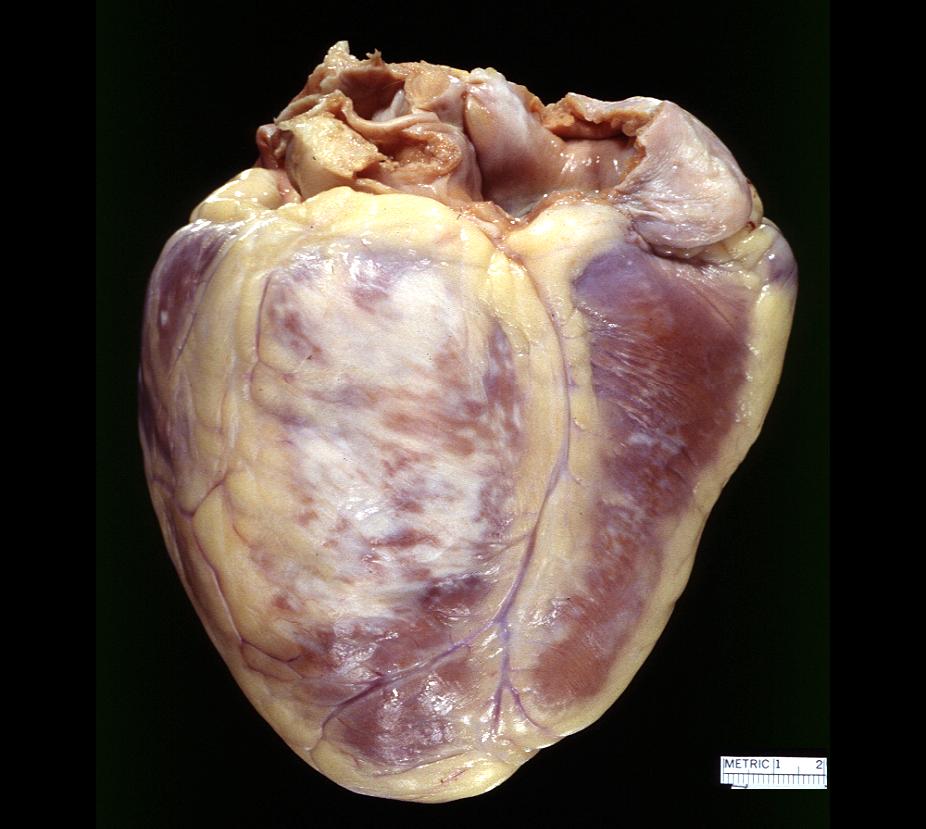
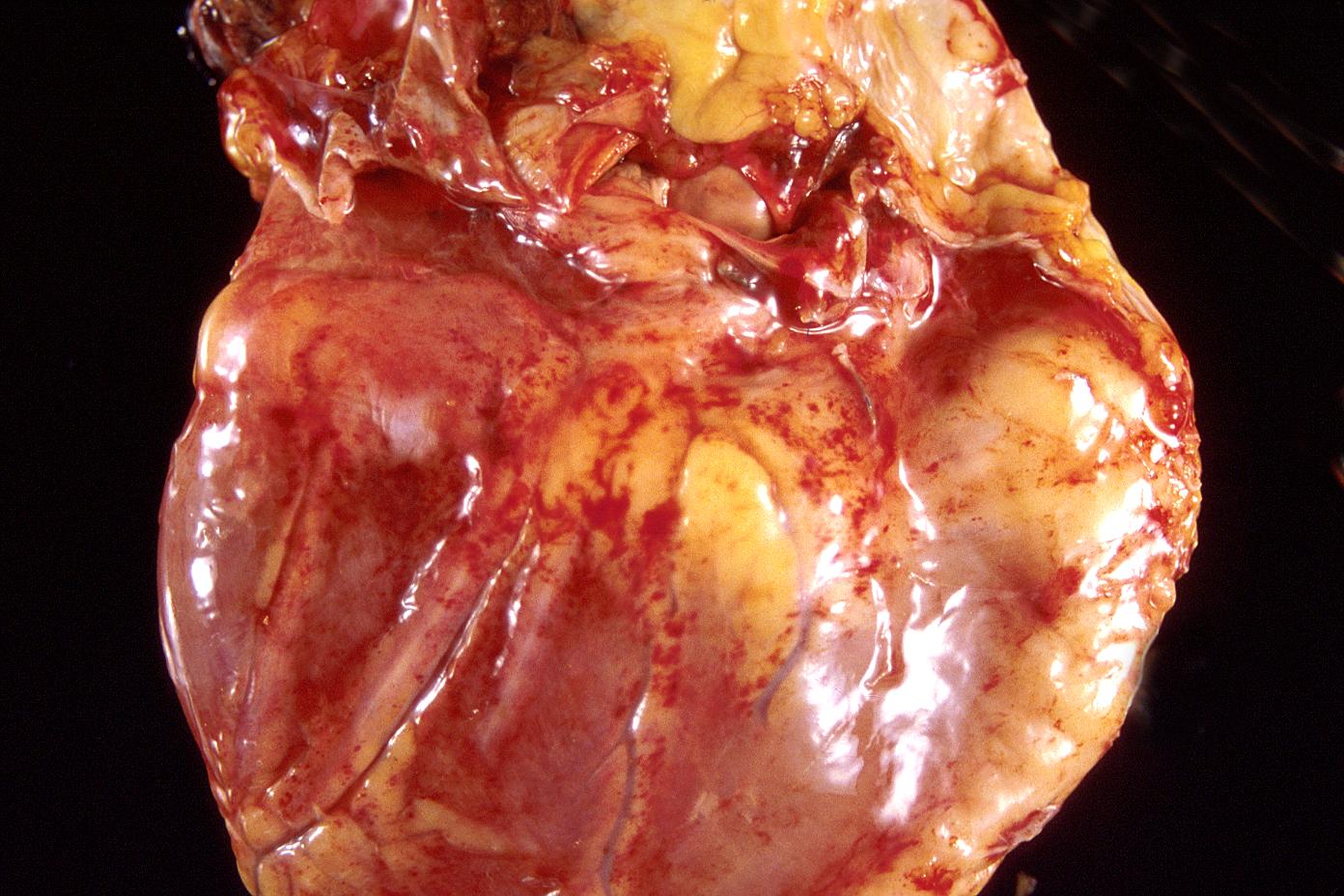
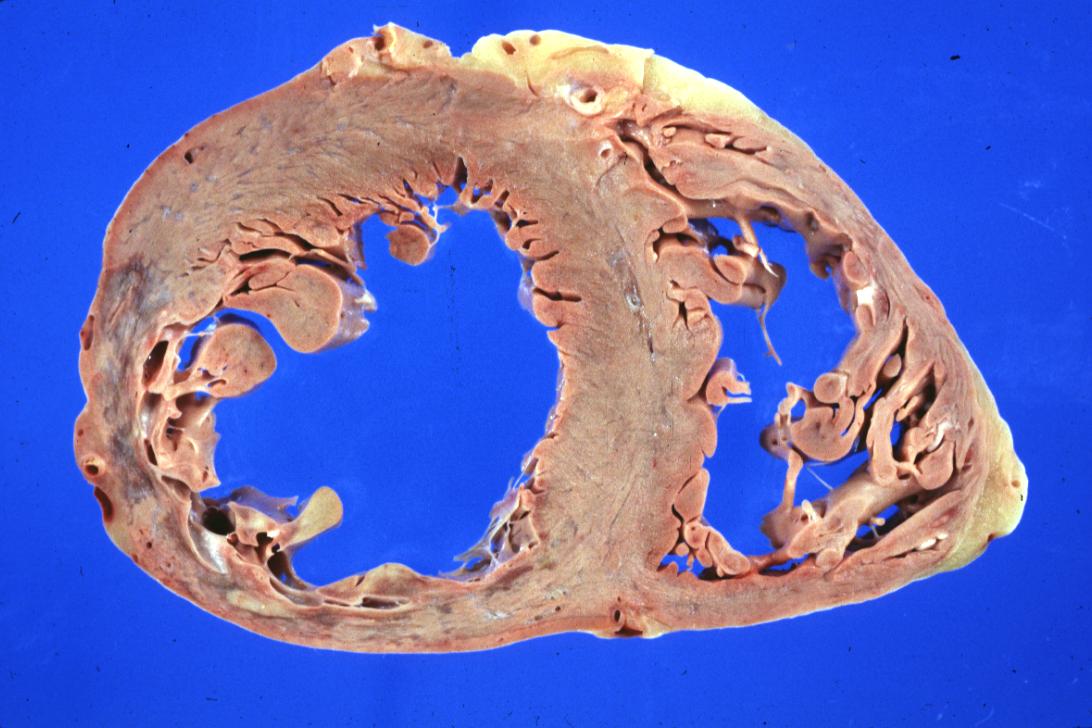
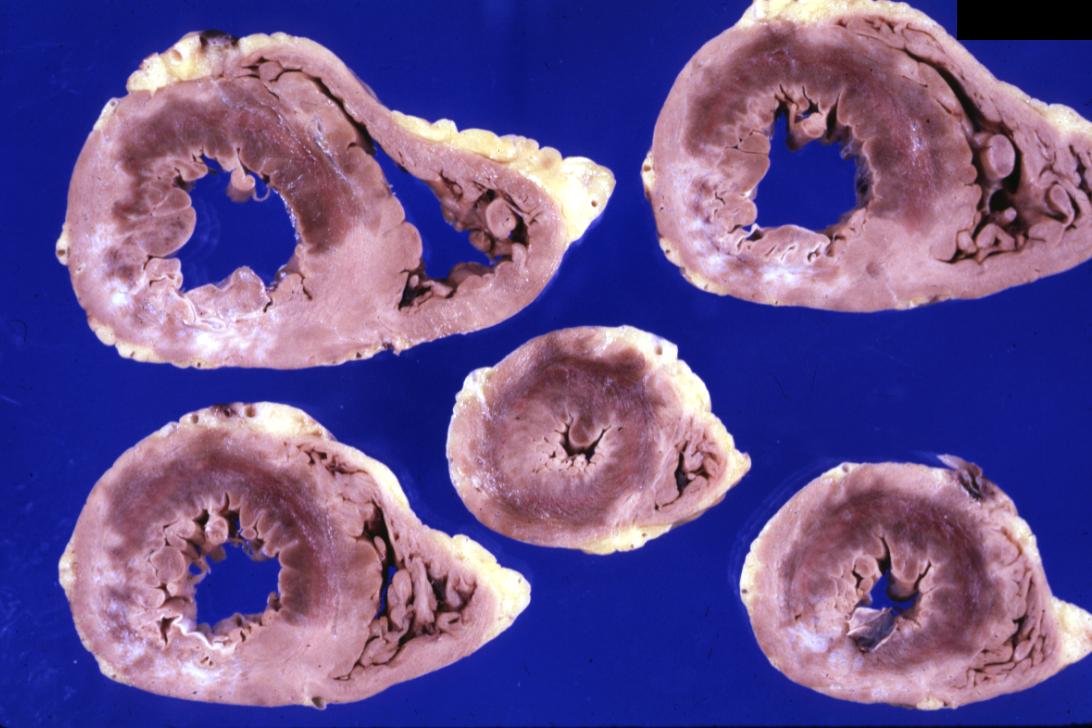
Gross example of acute infarction in fixed heart. Lesion is reflow necrosis stone heart also has old scar
-
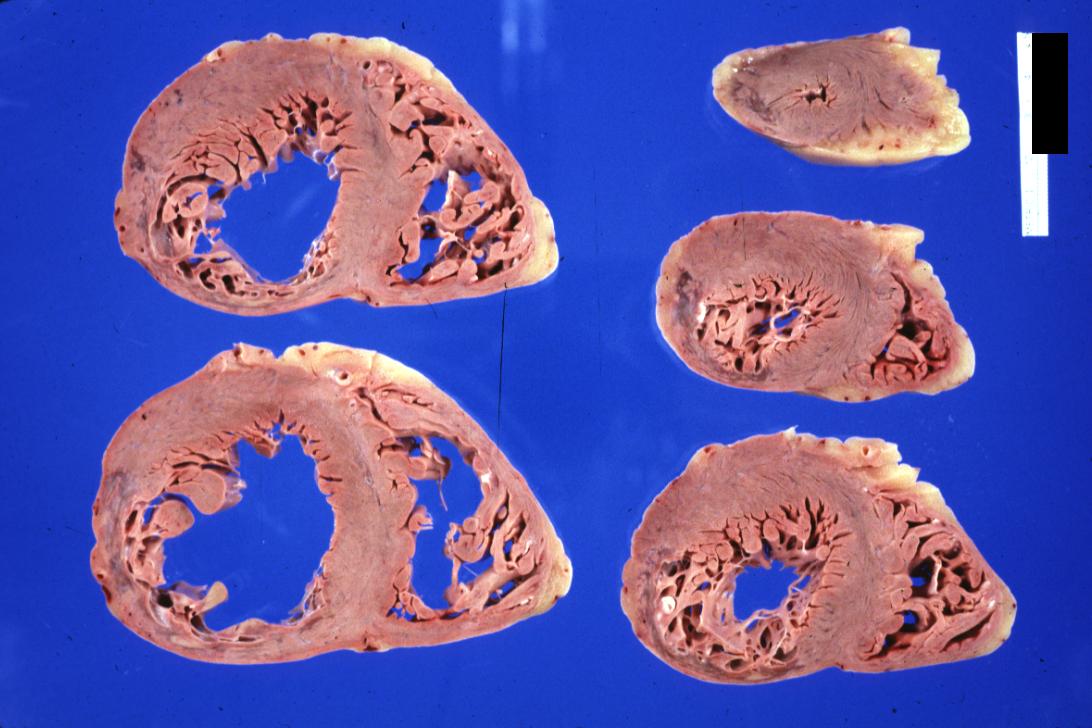
Gross example of acute infarction in fixed heart. Lesion is reflow necrosis stone heart also has old scar. Multisliced view.
-
Myocardial infarction, subacute phase.
-
Myocardial infarction, subacute, granulation tissue.
-
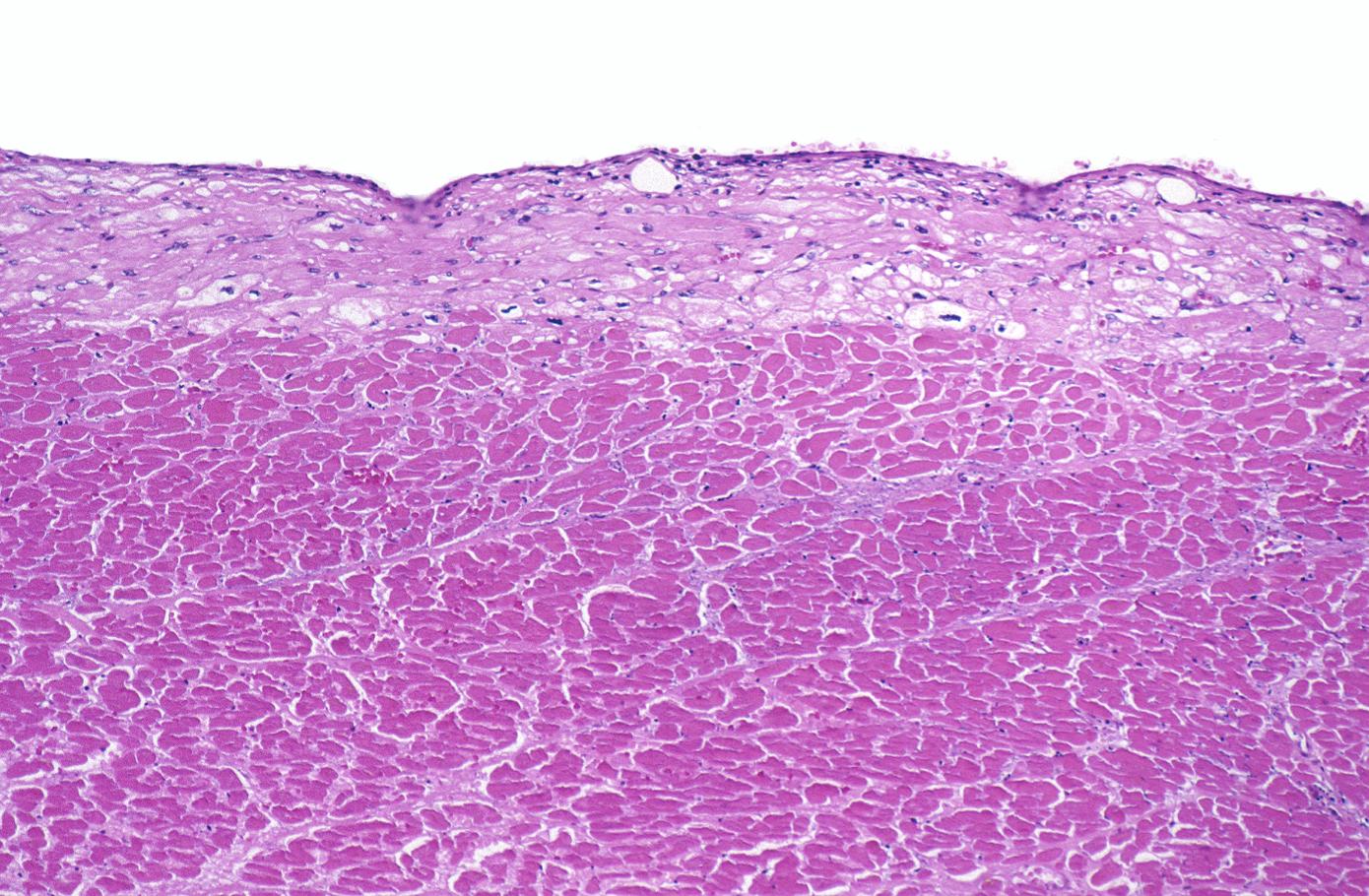
Myocardial infarction, subendocardial ischemia with swollen myocytes
-
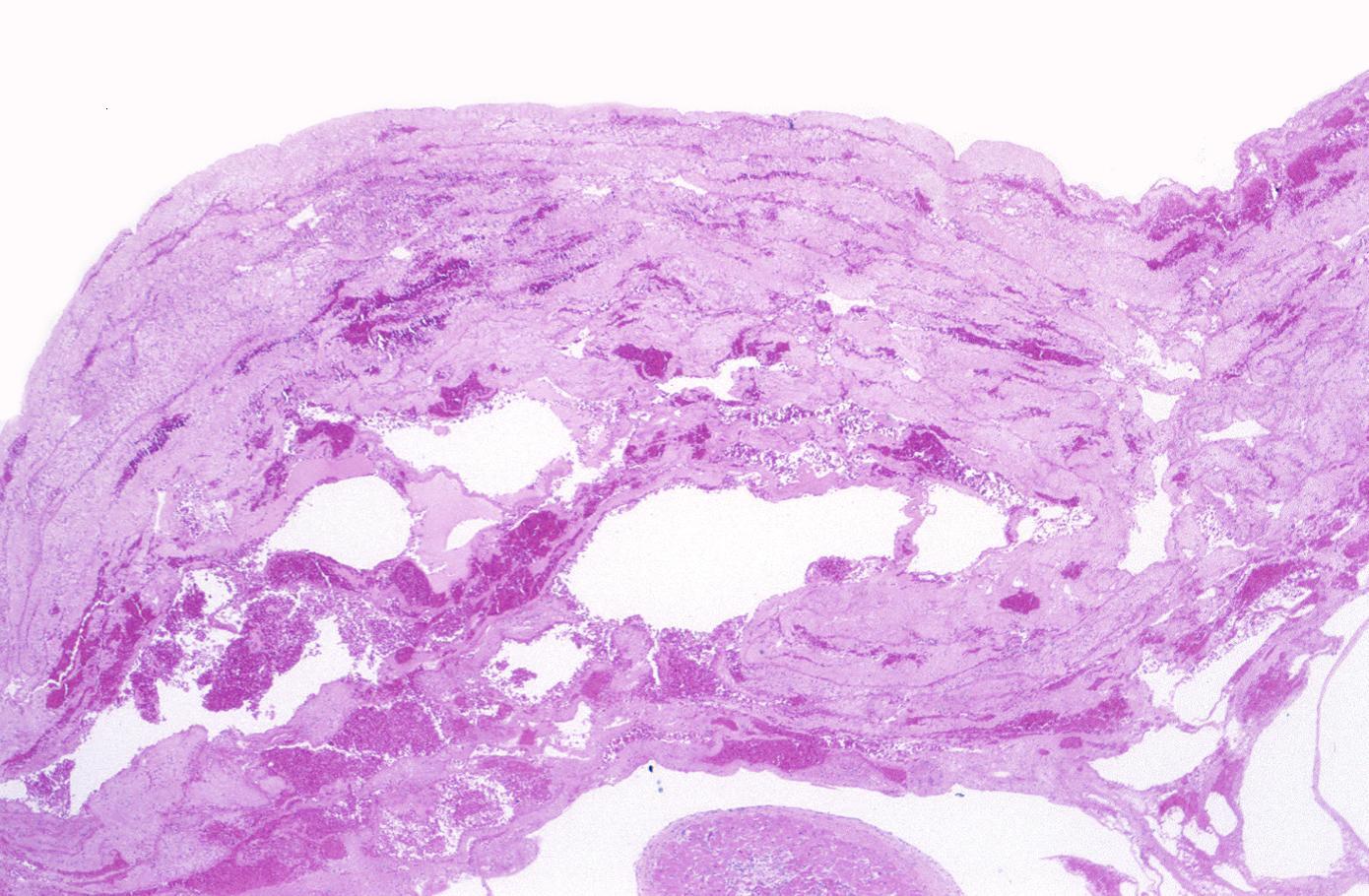
Myocardial infarction, mural thrombus
-
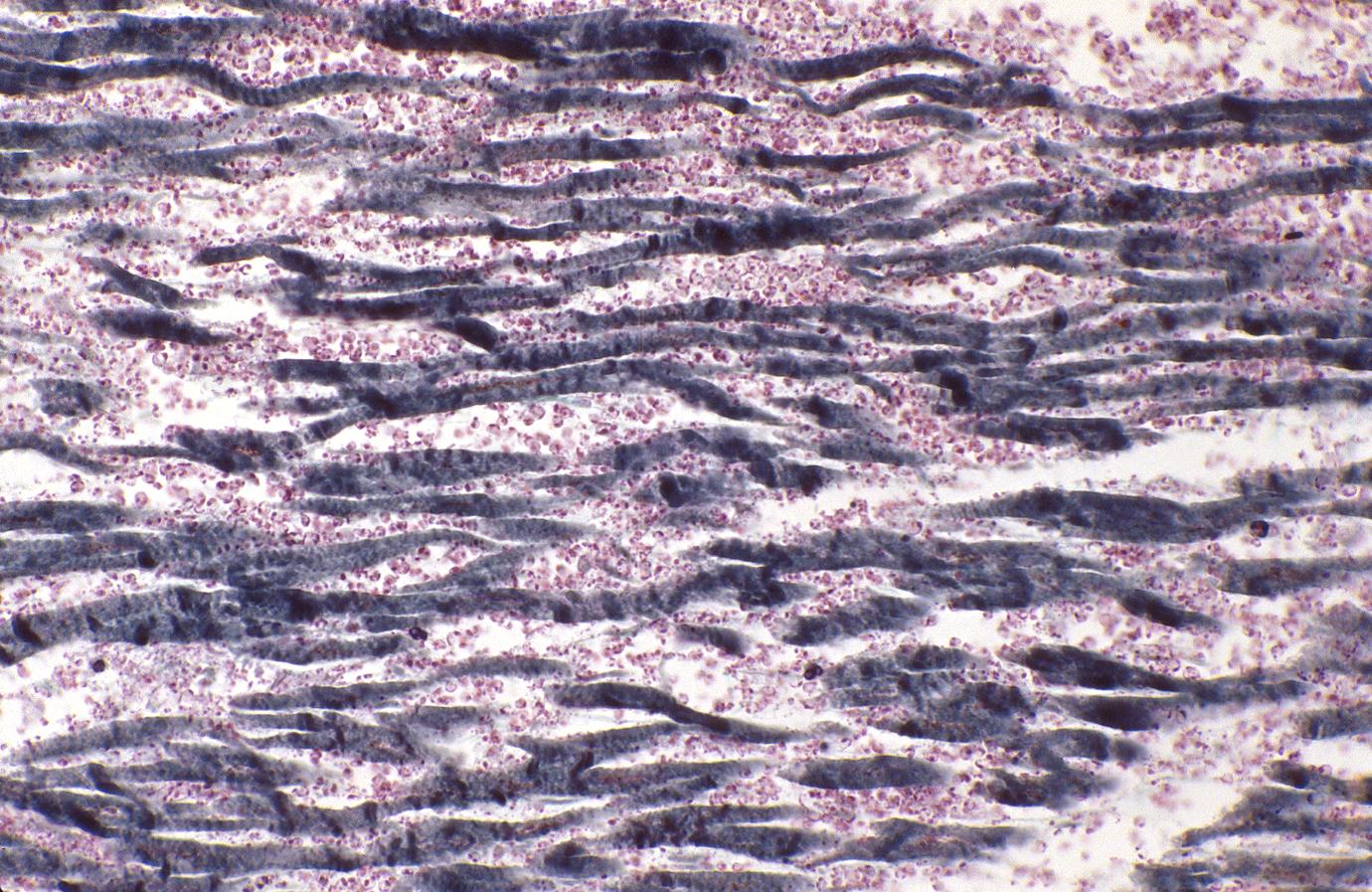
Acute myocardial infarction, ischemic fibers demonstrated by aldehyde fuchsin stain
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 Thygesen K, Alpert JS, White HD (2007). "Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction Joint ESC/ACCF/AHA/WHF". Circulation. 2007: 2634–2653. PMID 17951284.
- ↑ 2.0 2.1 2.2 2.3 Alpert JS, Thygesen K, Antman E, Bassand JP. (2000). "Myocardial infarction redefined-a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction". J Am Coll Cardiol. 37: 959–969. PMID 10987628.
- ↑ Wong CK, French JK, Aylward PE; et al. (2005). "Patients with prolonged ischemic chest pain and presumed-new left bundle branch block have heterogeneous outcomes depending on the presence of ST-segment changes". J. Am. Coll. Cardiol. 46 (1): 29–38. doi:10.1016/j.jacc.2005.02.084. PMID 15992631. Unknown parameter
|month=ignored (help) - ↑ Sgarbossa EB, Pinski SL, Barbagelata A; et al. (1996). "Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators". N. Engl. J. Med. 334 (8): 481–7. PMID 8559200. Unknown parameter
|month=ignored (help) - ↑ Macfarlane PW (2001). "Age, sex, and the ST amplitude in health and disease". J Electrocardiol. 34 Suppl: 235–41. PMID 11781962.
- ↑ Jaffe AS, Ravkilde J, Roberts R; et al. (2000). "It's time for a change to a troponin standard". Circulation. 102 (11): 1216–20. PMID 10982533. Unknown parameter
|month=ignored (help) - ↑ Morrow DA, Cannon CP, Jesse RL; et al. (2007). "National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes". Circulation. 115 (13): e356–75. doi:10.1161/CIRCULATIONAHA.107.182882. PMID 17384331. Unknown parameter
|month=ignored (help) - ↑ Jaffe AS, Babuin L, Apple FS (2006). "Biomarkers in acute cardiac disease: the present and the future". J. Am. Coll. Cardiol. 48 (1): 1–11. doi:10.1016/j.jacc.2006.02.056. PMID 16814641. Unknown parameter
|month=ignored (help) - ↑ Jaffe AS (2006). "Chasing troponin: how low can you go if you can see the rise?". J. Am. Coll. Cardiol. 48 (9): 1763–4. doi:10.1016/j.jacc.2006.08.006. PMID 17084246. Unknown parameter
|month=ignored (help) - ↑ Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH (2007). "National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: Analytical issues for biochemical markers of acute coronary syndromes". Circulation. 115 (13): e352–5. doi:10.1161/CIRCULATIONAHA.107.182881. PMID 17384332. Unknown parameter
|month=ignored (help) - ↑ Macrae AR, Kavsak PA, Lustig V; et al. (2006). "Assessing the requirement for the 6-hour interval between specimens in the American Heart Association Classification of Myocardial Infarction in Epidemiology and Clinical Research Studies". Clin. Chem. 52 (5): 812–8. doi:10.1373/clinchem.2005.059550. PMID 16556688. Unknown parameter
|month=ignored (help) - ↑ Wang K, Asinger RW, Marriott HJ (2003). "ST-segment elevation in conditions other than acute myocardial infarction". N. Engl. J. Med. 349 (22): 2128–35. doi:10.1056/NEJMra022580. PMID 14645641. Unknown parameter
|month=ignored (help) - ↑ Zimetbaum PJ, Josephson ME (2003). "Use of the electrocardiogram in acute myocardial infarction". N. Engl. J. Med. 348 (10): 933–40. doi:10.1056/NEJMra022700. PMID 12621138. Unknown parameter
|month=ignored (help) - ↑ Gibson CM (2003). "Has my patient achieved adequate myocardial reperfusion?". Circulation. 108: 504–07. PMID 12900495.
- ↑ Korosoglou G, Labadze N, Hansen A; et al. (2004). "Usefulness of real-time myocardial perfusion imaging in the evaluation of patients with first time chest pain". Am. J. Cardiol. 94 (10): 1225–31. doi:10.1016/j.amjcard.2004.07.104. PMID 15541235. Unknown parameter
|month=ignored (help) - ↑ Medrano R, Lowry RW, Young JB; et al. (1996). "Assessment of myocardial viability with 99mTc sestamibi in patients undergoing cardiac transplantation. A scintigraphic/pathological study". Circulation. 94 (5): 1010–7. PMID 8790039. Unknown parameter
|month=ignored (help) - ↑ Dakik HA, Howell JF, Lawrie GM; et al. (1997). "Assessment of myocardial viability with 99mTc-sestamibi tomography before coronary bypass graft surgery: correlation with histopathology and postoperative improvement in cardiac function". Circulation. 96 (9): 2892–8. PMID 9386154. Unknown parameter
|month=ignored (help) - ↑ Klein C, Nekolla SG, Bengel FM; et al. (2002). "Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography". Circulation. 105 (2): 162–7. PMID 11790695. Unknown parameter
|month=ignored (help) - ↑ Wagner A, Mahrholdt H, Holly TA; et al. (2003). "Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study". Lancet. 361 (9355): 374–9. doi:10.1016/S0140-6736(03)12389-6. PMID 12573373. Unknown parameter
|month=ignored (help) - ↑ Gosalia A, Haramati LB, Sheth MP, Spindola-Franco H (2004). "CT detection of acute myocardial infarction". AJR Am J Roentgenol. 182 (6): 1563–6. PMID 15150010. Unknown parameter
|month=ignored (help) - ↑ Lima JA (2003). "Myocardial viability assessment by contrast-enhanced magnetic resonance imaging". J. Am. Coll. Cardiol. 42 (5): 902–4. PMID 12957440. Unknown parameter
|month=ignored (help) - ↑ Isbell DC, Kramer CM (2005). "Cardiovascular magnetic resonance: structure, function, perfusion, and viability". J Nucl Cardiol. 12 (3): 324–36. PMID 15944538.
- ↑ Apple FS, Murakami MM (2005). "Cardiac troponin and creatine kinase MB monitoring during in-hospital myocardial reinfarction". Clin. Chem. 51 (2): 460–3. doi:10.1373/clinchem.2004.042887. PMID 15563477. Unknown parameter
|month=ignored (help) - ↑ Savage RM, Wagner GS, Ideker RE, Podolsky SA, Hackel DB (1977). "Correlation of postmortem anatomic findings with electrocardiographic changes in patients with myocardial infarction: retrospective study of patients with typical anterior and posterior infarcts". Circulation. 55 (2): 279–85. PMID 832343. Unknown parameter
|month=ignored (help) - ↑ Horan LG, Flowers NC, Johnson JC (1971). "Significance of the diagnostic Q wave of myocardial infarction". Circulation. 43 (3): 428–36. PMID 5544988. Unknown parameter
|month=ignored (help) - ↑ 26.0 26.1 Pahlm US, Chaitman BR, Rautaharju PM, Selvester RH, Wagner GS. (1998). "Comparison of the various electrocardiographic scoring codes for estimating anatomically documented sizes of single and multiple infarcts of the left ventricle". Am J Cardiol. 81: 809–15. PMID 9555767.
- ↑ Porela P, Helenius H, Pulkki K, Voipio-Pulkki LM (1999). "Epidemiological classification of acute myocardial infarction: time for a change?". Eur. Heart J. 20 (20): 1459–64. doi:10.1053/euhj.1998.1529. PMID 10493844. Unknown parameter
|month=ignored (help) - ↑ Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM (1998). "The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression". J Electrocardiol. 31 (3): 157–87. PMID 9682893. Unknown parameter
|month=ignored (help) - ↑ Ricciardi MJ, Davidson CJ, Gubermikoff G, Beohar N, Eckman LJ, Parker MA, Bonow RO. (2003). "Troponin I elevation and cardiac events after percutaneous coronary intervention". Am Heart J. 145: 522–28. PMID 12660677.
- ↑ 30.0 30.1 Noora J, Ricci C, Hastings D, Hills S, Cybulsky I. (1998). "Determination of troponin I release after CABG surgery". J Card Surg. 20: 129–35. PMID 15725136.
- ↑ Yokoyama Y, Chaitman BR, Hardison RM; et al. (2000). "Association between new electrocardiographic abnormalities after coronary revascularization and five-year cardiac mortality in BARI randomized and registry patients". Am. J. Cardiol. 86 (8): 819–24. PMID 11024394. Unknown parameter
|month=ignored (help) - ↑ Akkerhuis KM, Alexander JH, Tardiff BE; et al. (2002). "Minor myocardial damage and prognosis: are spontaneous and percutaneous coronary intervention-related events different?". Circulation. 105 (5): 554–6. PMID 11827918. Unknown parameter
|month=ignored (help) - ↑ 33.0 33.1 33.2 Herrman J. (2005). "Peri-procedural myocardial injury: 2005 update". Eur Heart J. 26: 2493–2519. PMID 16176941.
- ↑ Ricciardi MJ, Wu E, Davidson CJ; et al. (2001). "Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation". Circulation. 103 (23): 2780–3. PMID 11401931. Unknown parameter
|month=ignored (help) - ↑ Gustavsson CG, Hansen O, Frennby B (2004). "Troponin must be measured before and after PCI to diagnose procedure-related myocardial injury". Scand. Cardiovasc. J. 38 (2): 75–9. doi:10.1080/14017430410026755. PMID 15204231. Unknown parameter
|month=ignored (help) - ↑ Miller WL, Garratt KN, Burrit MF, Lennon RJ, Reeder GS, Jaffe AS. (2006). "Baseline troponin level: key to understanding the importance of post-PCI troponin elevations". Eur Heart J. 27: 1061–69. PMID 16481332.
- ↑ 37.0 37.1 Kovacevic R, Majkic-Singh N, Ignjatovic S, Otasevic P, Obrenovic R, Paris M, Vilotijevic B, Guermonprez JL. (2004). "Troponin T levels in detection of perioperative myocardial infarction after coronary artery bypass surgery". Clin Lab. 50: 437–45. PMID 15330513.
- ↑ Benoit MO, Paris M, Silleran J, Fiemeyer A, Moatti N (2001). "Cardiac troponin I: its contribution to the diagnosis of perioperative myocardial infarction and various complications of cardiac surgery". Crit. Care Med. 29 (10): 1880–6. PMID 11588444. Unknown parameter
|month=ignored (help) - ↑ Costa MA, Carere RG, Lichtenstein SV; et al. (2001). "Incidence, predictors, and significance of abnormal cardiac enzyme rise in patients treated with bypass surgery in the arterial revascularization therapies study (ARTS)". Circulation. 104 (22): 2689–93. PMID 11723020. Unknown parameter
|month=ignored (help) - ↑ Klatte K, Chaitman BR, Theroux P; et al. (2001). "Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial". J. Am. Coll. Cardiol. 38 (4): 1070–7. PMID 11583884. Unknown parameter
|month=ignored (help) - ↑ Brener SJ, Lytle BW, Schneider JP, Ellis SG, Topol EJ (2002). "Association between CK-MB elevation after percutaneous or surgical revascularization and three-year mortality". J. Am. Coll. Cardiol. 40 (11): 1961–7. PMID 12475456. Unknown parameter
|month=ignored (help) - ↑ Januzzi JL, Lewandrowski K, MacGillivray TE; et al. (2002). "A comparison of cardiac troponin T and creatine kinase-MB for patient evaluation after cardiac surgery". J. Am. Coll. Cardiol. 39 (9): 1518–23. PMID 11985917. Unknown parameter
|month=ignored (help) - ↑ Croal BL, Hillis GS, Gibson PH; et al. (2006). "Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery". Circulation. 114 (14): 1468–75. doi:10.1161/CIRCULATIONAHA.105.602370. PMID 17000912. Unknown parameter
|month=ignored (help) - ↑ Peels C, Visser CA, Kupper AJ, Visser FC, Roos JP. Usefulness of two-dimensional echocardiography for immediate detection of myocardial ischemia in the emergency room. Am J Cardiol. 1990; 65: 687–91. PMID 2316447
- ↑ Sabia P, Abbott RD, Afrookteh A, Keller MW, Touchstone DA, Kaul S. Importance to two-dimensional echocardiographic assessment of left ventricular systolic function in patients presenting to the emergency room with cardiac-related symptoms. Circulation. 1991; 84:1615–24. PMID 1914101
- ↑ Saeian K, Rhyne TL, Sagar KB. Ultrasonic tissue characterization for diagnosis of acute myocardial infarction in the coronary care unit. Am J Cardiol. 1994; 74: 1211–15. PMID 7977092
- ↑ Tatum JL, Jesse RL, Kontos MC, Nicholson CS, Schmidt KL, Roberts CS, Ornato JP. Comprehensive strategy for the evaluation and triage of the chest pain patients. Ann Emerg Med. 1997; 29:116–25. PMID 8998090
- ↑ Kim RJ, Fieno DS, Parrish TB, Harris K, Chen E-L, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible necrosis, infarct age, and contractile function. Circulation. 1999;100:1992–2002. PMID 10556226
- ↑ Appelbaum E, Kirtane AJ, Clark A, Pride YB, Gelfand EV, Harrigan CJ, Kissinger KV, Manning WJ, Gibson CM. Association of TIMI Myocardial Perfusion Grade and ST-segment resolution with cardiovascular magnetic resonance measures of microvascular obstruction and infarct size following ST-segment elevation myocardial infarction. J Thromb Thrombolysis. 2008 Feb 2; PMID 18246410
- ↑ Salomaa V, Koukkunen H, Ketonen M, Immonen-Räihä P , Kärjä-Koskenkari P, Mustonen J, Lehto S, Torppa J, Lehtonen A, Tuomilehto J, Kesäniemi YA, Pyörälä K. A new definition for myocardial infarction: what difference does it make? Eur Heart J. 2005; 27: 1719–25. PMID 15814567
- ↑ Roger VL, Killian JM, Weston SA, Jaffe AS, Kors J, Santrach PJ, Tunstall-Pedoe H, Jacobsen SJ. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation. 2006;114: 790–97. PMID 16908764