Carbon monoxide poisoning: Difference between revisions
No edit summary |
|||
| (46 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{{CMG}} | {{CMG}}; {{AE}} {{ADI}}, {{FA}} | ||
{{SI}} | {{SI}} | ||
==Overview== | ==Overview== | ||
Carbon monoxide poisoning occurs after the inhalation of [[carbon monoxide]] gas. Carbon monoxide (CO) is a product of combustion of organic matter under conditions of restricted oxygen supply, which prevents complete oxidation to [[carbon dioxide]] (CO<sub>2</sub>). Carbon monoxide is colorless, odorless, tasteless, and non-irritating, making it difficult to detect. Carbon monoxide is a significantly toxic gas with poisoning being the most common type of fatal poisoning in many countries.<ref name="Toxicology2002-omaye">{{cite journal | author=Omaye ST. | title=Metabolic modulation of carbon monoxide toxicity | journal=Toxicology | year=2002 | pages=139-50 | volume=180 | issue=2 | id=PMID 12324190}}</ref> Symptoms of mild poisoning include headaches and [[flu|flu-like]] effects; larger exposures can lead to significant toxicity of the [[central nervous system]] and [[heart]]. Following poisoning, long-term [[sequela|sequelae]] often occur. [[Carbon monoxide]] can also have severe effects on the [[fetus]] of a pregnant woman. The mechanisms by which carbon monoxide produces toxic effects are not yet fully understood, but [[hemoglobin]], [[myoglobin]], and [[mitochondrial]] [[cytochrome oxidase]] are thought to be compromised. Treatment largely consists of administering 100% [[oxygen]] or [[hyperbaric oxygen]] therapy, although the optimum treatment remains controversial.<ref name="ToxicolRev2005-buckley">{{cite journal | author=Buckley NA, Isbister GK, Stokes B, Juurlink DN. | title=Hyperbaric oxygen for carbon monoxide poisoning : a systematic review and critical analysis of the evidence | journal=Toxicol Rev | year=2005 | pages=75-92 | volume=24 | issue=2 | id=PMID 16180928}}</ref> Domestic carbon monoxide poisoning can be prevented by the use of household [[carbon monoxide detector]]s. | |||
==Historical perspective== | |||
*Carbon monoxide poisoning may manifest as [[lethargy]], [[fatigue]] and [[tiredness]], [[depression]], [[memory loss]], emotional disturbance, and [[Hallucinations|visual/auditory hallucinations]]. | |||
*In a few cases, it was associated with 'haunted houses' where the residents experienced strange sounds and visions, feelings of dread, sudden onset illness, and death of all the members in the house. | |||
*In 1921, Dr. William Wilmer, an ophthalmologist published a case in American journal of ophthalmology in which he described the experiences of a family with similar symptoms as mentioned above.<ref>[http://www.ghostvillage.com/resources/2004/resources_10312004.shtml]</ref> | |||
*In 2005, a report was published describing a young female of age 23, found [[Delirium|delirious]] and [[Hyperventilation|hyperventilating]], as a result of leakage and accumulation of gas from the heater. She believed to have seen a 'ghost' in the shower. <ref>Jiann-Ruey Ong, Sheng-Wen Hou, Hsien-Tsung Shu, Huei-Tsair Chen, and Chee-Fah Chong. Diagnostic pitfall: carbon monoxide poisoning mimicking hyperventilation syndrome. ''The [[American Journal of Emergency Medicine]]'' | |||
Volume 23, Issue 7 , November 2005, Pages 903-904</ref> | |||
==Sources== | ==Sources== | ||
* Main sources of CO that are responsible for environmental pollution are house hold fires, heaters, furnaces, motor vehicle exhaust, propane fueled equipment (such as ice resurfacers, forklifts), engine-driven generators, and wood burning stove.<ref>{{cite journal |author=Johnson C, Moran J, Paine S, Anderson H, Breysse P |title=Abatement of toxic levels of carbon monoxide in Seattle ice-skating rinks |journal=Am J Public Health |volume=65 |issue=10 |pages=1087-90 |year=1975 |id=PMID 1163706}}</ref><ref>{{cite journal |author=Fawcett T, Moon R, Fracica P, Mebane G, Theil D, Piantadosi C |title=Warehouse workers' headache. Carbon monoxide poisoning from propane-fueled forklifts |journal=J Occup Med |volume=34 |issue=1 |pages=12-5 |year=1992 |id=PMID 1552375}}</ref> | |||
* CO poisoning can also occur in scuba diving due to malfunctioning of [[diving air compressor]]s. | |||
* Another source is exposure to the organic solvent [[methylene chloride]], which is metabolized to CO by the body.<ref name="DrugMetabDispos1975-kubic">{{cite journal | author=Kubic VL, Anders MW. | title=Metabolism of dihalomethanes to carbon monoxide. II. In vitro studies | journal=Drug Metab Dispos | year=1975 | pages=104-12 | volume=3 | issue=2 | id=PMID 236156}}</ref> | |||
* Polluted air often contains unhealthy levels of carbon monoxide. Many areas of the US have struggled to achieve legislated limits. Significant advances have been made since the implementation by 1990 of a vehicle emissions limit of 3.4 gpm (grams per mile), a large reduction from the previous limit of 87 gpm.<ref name="urlCarbon Monoxide (CO) Pollution in Outdoor Air | US EPA">{{cite web |url=https://www.epa.gov/co-pollution |title=Carbon Monoxide (CO) Pollution in Outdoor Air | US EPA |format= |work= |accessdate=}}</ref> | |||
==Pathophysiology == | |||
The precise mechanisms by which toxic effects are induced by CO are not fully understood. It may damage the body in one of the following ways:<ref name="pmid4502938">{{cite journal| author=Brunori M, Bonaventura J, Bonaventura C, Antonini E, Wyman J| title=Carbon monoxide binding by hemoglobin and myoglobin under photodissociating conditions. | journal=Proc Natl Acad Sci U S A | year= 1972 | volume= 69 | issue= 4 | pages= 868-71 | pmid=4502938 | doi= | pmc=426583 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4502938 }}</ref> | |||
* Binds to [[hemoglobin]] thus reducing [[oxygen transportation]] in blood<ref name="pmid29461288">{{cite journal| author=Culnan DM, Craft-Coffman B, Bitz GH, Capek KD, Tu Y, Lineaweaver WC et al.| title=Carbon Monoxide and Cyanide Poisoning in the Burned Pregnant Patient: An Indication for Hyperbaric Oxygen Therapy. | journal=Ann Plast Surg | year= 2018 | volume= | issue= | pages= | pmid=29461288 | doi=10.1097/SAP.0000000000001351 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29461288 }}</ref> | |||
* Binds with [[myoglobin]] to decrease its oxygen storage capacity<ref name="pmid7638619">{{cite journal| author=Lim M, Jackson TA, Anfinrud PA| title=Binding of CO to myoglobin from a heme pocket docking site to form nearly linear Fe-C-O. | journal=Science | year= 1995 | volume= 269 | issue= 5226 | pages= 962-6 | pmid=7638619 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7638619 }}</ref> | |||
* Reacts with mitochondrial [[cytochrome oxidase]] (specifically [[cytochrome C]]) hence inhibiting [[cellular respiration]].<ref name="pmid12969439">{{cite journal| author=Alonso JR, Cardellach F, López S, Casademont J, Miró O| title=Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. | journal=Pharmacol Toxicol | year= 2003 | volume= 93 | issue= 3 | pages= 142-6 | pmid=12969439 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12969439 }}</ref> | |||
===CO and Hemoglobin === | |||
[ | * [[Carbon monoxide]] (CO) has a significant affinity to the iron sites in [[hemoglobin]], the principal oxygen-carrying compound in [[blood]]. The affinity between [[carbon monoxide]] and [[hemoglobin]] is 240 times stronger than the affinity between [[hemoglobin]] and [[oxygen]]. | ||
[ | * CO binds to [[hemoglobin]], producing [[carboxyhemoglobin]] ([[Carboxyhemoglobin|COHb]]) - the traditional belief is that [[carbon monoxide]] toxicity arises from the formation of [[carboxyhemoglobin]], which decreases the oxygen-carrying capacity of the [[blood]]. This inhibits the transport, delivery, and utilization of [[oxygen]].<ref name="JPhysiol1895-Haldane">{{cite journal | author=Haldane J. | title=The action of carbonic oxide on man | journal=J Physiol | year=1895 | pages=430-62 | volume=18}} | ||
</ref> | |||
== | * Because hemoglobin is a [[tetramer]] with four [[oxygen]] binding sites, binding of CO at one of these sites also increases the [[oxygen]] affinity of the remaining 3 sites, which interferes with normal release of [[oxygen]]. This causes [[hemoglobin]] to retain [[oxygen]] that would otherwise be delivered to the tissue.<ref name="Toxicology2003-Gorman">{{cite journal | author=Gorman D, Drewry A, Huang YL, Sames C. | title=The clinical toxicology of carbon monoxide | journal=Toxicology | year=2003 | pages=25-38 | volume=187 | issue=1 | id=PMID 12679050}}</ref> | ||
* Amount of [[oxygen]] available for tissue use are decreased. This situation is described as CO shifting the [[oxygen-hemoglobin dissociation curve|oxygen dissociation curve]] to the left. Blood oxygen content is actually increased in the case of carbon monoxide poisoning; because all the oxygen is in the blood, none is being given to the tissues, and this causes [[Tissue hypoxia|tissue hypoxic injury]]. | |||
[[File:Hemoglobin saturation curve.svg.png|thumb|left|369x369px|CO dissociation curve [https://commons.wikimedia.org/wiki/File:Hemoglobin_saturation_curve.svg Source:Case courtesy of By Hazmat2, via Wikimedia Commons]]] | |||
<br clear="left" /> | |||
=== | === CO and Myoglobin === | ||
* CO has 60 times greater affinity to [[myoglobin]] as compared to [[oxygen]]. It can bind readily to [[myoglobin]], which is another heme-containing protein, abundantly present in the muscles of body. | |||
* Binding of CO to [[myoglobin]] markedly reduces its [[oxygen]] carrying capacity, leading to decrease in [[cardiac output]], [[hypotension]] which ultimately resulting in [[cerebral ischemia]]. | |||
* CO bound to [[myolobin]] is later released in the body, which subsequently binds with the [[hemoglobin]]. This phenomenon is responsible for delayed return of symptoms in CO poisoning. <ref name="pmid123241902">{{cite journal| author=Omaye ST| title=Metabolic modulation of carbon monoxide toxicity. | journal=Toxicology | year= 2002 | volume= 180 | issue= 2 | pages= 139-50 | pmid=12324190 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12324190 }}</ref> | |||
=== CO and Mitochondrial Cytochrome Oxidase C === | |||
* CO has lesser affinity with [[cytochrome c oxidase]] in [[mitochondria]] than [[oxygen]] so the damage occurs only in the setting of significant [[hypoxia]]. | |||
* CO damages the cell by interfering with [[aerobic metabolism]] and synthesis of [[ATP]], which leads to shifting of metabolism to [[anaerobic]] and accumulation of [[lactic acid]] [[Intracellular|intracellularly]] with significant [[anoxia]]. This ultimately leads to [[necrosis]] and [[cell death]]. | |||
* Once CO is bound to [[cytochrome c oxidase]], the rate of dissociation is very slow. Hence, causing prolonged damage to oxidaive metabolism in the cell.<ref name="pmid11387414">{{cite journal| author=Blumenthal I| title=Carbon monoxide poisoning. | journal=J R Soc Med | year= 2001 | volume= 94 | issue= 6 | pages= 270-2 | pmid=11387414 | doi= | pmc=1281520 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11387414 }}</ref> | |||
==Epidemiology & Demographics== | |||
* Carbon monoxide poisoning is the most common type of fatal poisoning in France and the United States. It has been estimated that more than 40,000 people per year seek medical attention for carbon monoxide poisoning in the United States.<ref name="J Emerg Med1998-hampson">{{cite journal | author=Hampson NB. | title=Emergency department visits for carbon monoxide poisoning in the Pacific Northwest | journal=J Emerg Med | year=1998 | pages=695-8 | volume=16 | issue=5 | id=PMID 9752939}}</ref> | |||
* In many industrialized countries, [[carbon monoxide]] may be the cause of greater than 50% of fatal poisonings. | |||
* In the U.S., about 200 people die each year from carbon monoxide poisoning associated with home fuel-burning heating equipment. The [[Centers for Disease Control and Prevention|CDC]] reports, "Each year, more than 500 Americans die from unintentional CO poisoning, and more than 2,000 commit suicide by intentionally poisoning themselves."<ref>[http://www.cdc.gov/co/faqs.htm]</ref> | |||
== | ===CO and Suicide=== | ||
* [[Carbon monoxide]] has emerged as a common mean of suicide by poisoning, since the placement of strict legal restrictions on poisons like [[cyanide]] and [[arsenic]]. | |||
* [[Suicide]] was also often committed by inhaling exhaust fumes of running car engines. In the past, [[Exhaust gas|motor car exhaust]] may have contained up to 25% carbon monoxide. However, newer cars have [[catalytic converter]]s, which can eliminate over 99% of carbon monoxide produced.<ref name="Chest1999-vossberg">{{cite journal | author=Vossberg B, Skolnick J. | title=The role of catalytic converters in automobile carbon monoxide poisoning: a case report | journal=Chest | year=1999 | pages=580-1 | volume=115 | issue=2 | id=PMID 10027464}}</ref> However, even cars with catalytic converters can produce substantial carbon monoxide if an idling car is left in an enclosed space. This is due to reduced oxygen availability, and therefore, less efficient combustion. | |||
* As carbon monoxide poisoning via car exhaust has become less of a suicide option, there has been an increase in new methods of carbon monoxide poisoning such as [[Charcoal-burning suicide|burning charcoal]] or other fossil fuels within a confined space, such as a small room, tent, or car.<ref name="PsychiatrServ2001-chung">{{cite journal | author=Chung WS, Leung CM. | title=Carbon monoxide poisoning as a new method of suicide in Hong Kong | journal=Psychiatr Serv | year=2001 | pages=836-7 | volume=52 | issue=6 | id=PMID 11376237}}</ref> | |||
==Toxicity== | |||
* Carbon monoxide is a significantly toxic gas, although patients may demonstrate varied clinical manifestations with different outcomes, even under similar exposure conditions.<ref name="Toxicology2000-raub">{{cite journal | author=Raub JA, Mathieu-Nolf M, Hampson NB, Thom SR. | title=Carbon monoxide poisoning-a public health perspective | journal=Toxicology | year=2000 | pages=1-14| volume=145 | issue=1 | id=PMID 10771127}}</ref> | |||
=== Risk factors === | |||
* Toxicity is also increased by several factors as follows: | |||
** Increased activity and [[Ventilation (physiology)|rate of ventilation]] | |||
** Pre-existing [[cerebral]] or [[cardiovascular disease]] | |||
** Reduced [[cardiac output]] | |||
** [[Anemia]] or other hematological disorders | |||
** Decreased [[barometric pressure]] | |||
** High [[metabolic rate]]. | |||
=== | === Classification: === | ||
* The degrees of poisoning have been described as mild, moderate, and severe based on carboxyhaemoglobin percentage levels and clinical symptoms.:<ref name="pmid19445736">{{cite journal| author=Olson K, Smollin C| title=Carbon monoxide poisoning (acute). | journal=BMJ Clin Evid | year= 2008 | volume= 2008 | issue= | pages= | pmid=19445736 | doi= | pmc=2907971 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19445736 }}</ref> | |||
** '''Mild carbon monoxide poisoning:''' [[Carboxyhaemoglobin]] level of over 10% without clinical signs or symptoms of carbon monoxide poisoning. | |||
** '''Moderate carbon monoxide poisoning:''' [[Carboxyhaemoglobin]] level of over 10%, but under 20-25%, with minor clinical signs and symptoms of poisoning, such as [[headache]], [[lethargy]], or [[fatigue]]. | |||
** '''Severe carbon monoxide poisoning:''' [[Carboxyhaemoglobin]] level of over 20-25%, [[loss of consciousness]], and [[confusion]] or signs of [[cardiac]] ischemia, or both. | |||
Carbon monoxide | * Levels of [[carbon monoxide]] bound in the [[blood]] can be determined by measuring [[carboxyhaemoglobin]], which is a stable complex of carbon monoxide and [[hemoglobin]] that forms in [[red blood cells]]. | ||
* [[Carbon monoxide]] also functions as a [[neurotransmitter]]. | |||
* Normal [[carboxyhemoglobin]] levels in an average person are less than 5%, whereas cigarette smokers (two packs/day) may have levels up to 9%.<ref name="Clinical toxicology2001-ford">{{cite book | editor = Ford MD, Delaney KA, Ling LJ, Erickson T. | title = Clinical toxicology | year = 2001 | publisher = WB Saunders Company | id = ISBN 0-7216-5485-1}}</ref> | |||
* Serious toxicity is often associated with [[carboxyhemoglobin]] levels above 25%, and the risk of fatality is high with levels over 70%. Still, no consistent dose response relationship has been found between [[carboxyhemoglobin]] levels and clinical effects.<ref name="JToxClinTox1994-hardy">{{cite journal | author=Hardy KR, Thom SR. | title=Pathophysiology and treatment of carbon monoxide poisoning | journal=J Toxicol Clin Toxicol | year=1994 | pages=613-29 | volume=32 | issue=6 | id=PMID 7966524}}</ref> | |||
* Hence, [[carboxyhemoglobin]] levels are better guide for exposure levels than effects as they do not reliably predict clinical course or short- or long-term outcome.<ref name="MedJAust1999-Scheinkestel">{{cite journal | author=Scheinkestel CD, Bailey M, Myles PS, Jones K, Cooper DJ, Millar IL, Tuxen DV. | title=Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: a randomised controlled clinical trial | journal=Med J Aust | year=1999 | pages=203-10 | volume=170 | issue=5 | id=PMID 10092916}}</ref> | |||
* The effects of carbon monoxide in parts per million are listed below:<ref name="pmid19022078">{{cite journal| author=Goldstein M| title=Carbon monoxide poisoning. | journal=J Emerg Nurs | year= 2008 | volume= 34 | issue= 6 | pages= 538-42 | pmid=19022078 | doi=10.1016/j.jen.2007.11.014 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19022078 }}</ref> | |||
**35 ppm (0.0035%): [[Headache]] and [[dizziness]] within six to eight hours of constant exposure. | |||
**100 ppm (0.01%): Slight [[headache]] in two to three hours. | |||
**200 ppm (0.02%): Slight [[headache]] within two to three hours. | |||
**400 ppm (0.04%): Frontal [[headache]] within one to two hours. | |||
**800 ppm (0.08%): [[Dizziness]], [[nausea]], and [[convulsions]] within 45 minutes. Insensible within two hours. | |||
**1,600 ppm (0.16%): [[Headache]], [[dizziness]], and [[nausea]] within 20 minutes. Death in less than two hours. | |||
**3,200 ppm (0.32%): [[Headache]], [[dizziness]] and [[nausea]] in five to ten minutes. Death within 30 minutes. | |||
**6,400 ppm (0.64%): [[Headache]] and [[dizziness]] in one to two minutes. Death in less than 20 minutes. | |||
**12,800 ppm (1.28%): [[Unconsciousness]] after 2-3 breaths. Death in less than three minutes. | |||
*In addition, a recent report concludes that carbon monoxide exposure can lead to a decrease in lifespan significantly as a result of damaged [[myocardium]].<ref>{{cite journal | author= Henry CR, Satran D, Lindgren B, Adkinson C, Nicholson CI, Henry TD, MD| title= Myocardial Injury and Long-term Mortality Following Moderate to Severe Carbon Monoxide Poisoning|journal=JAMA | year=2006| volume=295| pages=398-402}} [http://jama.ama-assn.org/cgi/content/abstract/295/4/398 Abstract]</ref> | |||
==Signs and Symptoms== | |||
Carbon monoxide poisoning is particularly difficult to diagnose since it is not easily detected by human senses. Since CO poisoning is a diagnosis frequently overlooked, it is overly emphasized to measure [[carboxyhemoglobin]] in suspicious cases. The clinical manifestations include : | |||
===Acute === | |||
==== Most common: ==== | |||
Common symptoms of acute CO poisoning include:<ref name="pmid19617936">{{cite journal| author=Quinn DK, McGahee SM, Politte LC, Duncan GN, Cusin C, Hopwood CJ et al.| title=Complications of carbon monoxide poisoning: a case discussion and review of the literature. | journal=Prim Care Companion J Clin Psychiatry | year= 2009 | volume= 11 | issue= 2 | pages= 74-9 | pmid=19617936 | doi= | pmc=2707118 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19617936 }}</ref> | |||
* [[Headache]] | |||
* [[Tachycardia]] | |||
* | |||
* [[Hypertension]] | |||
* [[Dizziness]] | |||
* [[Confusion]] | |||
* [[Convulsions]] | |||
* [[Coma]] | |||
* [[Respiratory arrest]] | |||
=== | ==== Less common ==== | ||
Less common symptoms of acute CO poisoning are as follows:<ref name="JKoreanMedSci2001-choi">{{cite journal | author=Choi IS. | title=Carbon monoxide poisoning: systemic manifestations and complications | journal=J Korean Med Sci | year=2001 | pages=253-61 | volume=16 | issue=3 | id=PMID 11410684}}</ref> | |||
* [[Myocardial Ischemia|Myocardial ischemia]] | |||
* [[Atrial fibrillation]] | |||
* [[Pneumonia]] | |||
* [[Pulmonary edema]] | |||
* [[Hyperglycemia]] | |||
* [[Lactic acidosis]] | |||
* [[Muscle]] [[necrosis]] | |||
* [[Acute renal failure]] | |||
* [[Skin]] [[Lesion|lesions]] | |||
* [[Visual]] and [[auditory]] dysfunction | |||
=== Delayed === | |||
CO poisoning may cause delayed clinical manifestations in the form of severe neurological manifestations.These symptoms occur days or even weeks after an acute poisoning epispde. Common clinical manifestations include:<ref name="ClinNeurolNeurosurg2001-roohi">{{cite journal | author=Roohi F, Kula RW, Mehta N. | title=Twenty-nine years after carbon monoxide intoxication | journal=Clin Neurol Neurosurg | year=2001 | pages=92-5 | volume=103 | issue=2 | id=PMID 11516551}}</ref><ref name="pmid11410684">{{cite journal| author=Choi IS| title=Carbon monoxide poisoning: systemic manifestations and complications. | journal=J Korean Med Sci | year= 2001 | volume= 16 | issue= 3 | pages= 253-61 | pmid=11410684 | doi=10.3346/jkms.2001.16.3.253 | pmc=3054741 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11410684 }}</ref> | |||
* Difficulty with higher intellectual functions and [[short-term memory]] | |||
* [[Dementia]] | |||
* [[Irritability]] | |||
* [[Gait Abnormalities|Gait disturbance]] | |||
* [[Speech disturbances]] | |||
* [[Parkinson's disease|Parkinson-like syndromes]] | |||
* [[Cortical blindness]] | |||
* [[Depression]] | |||
=== | ===Chronic=== | ||
=== | Long term, repeat exposures present a greater risk to individuals with [[coronary heart disease]] and in pregnant patients.<ref name="N Engl J Med1989-allred">{{cite journal | author=Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J. | title=Short-term effects of carbon monoxide exposure on the exercise performance of subjects with coronary artery disease | journal=N Engl J Med | year=1989 | pages=1426-32 | volume=321 | issue=21 | id=PMID 2682242}}</ref> Chronic exposure may increase the incidence of cardiovascular symptoms in some workers, such as motor vehicle examiners, firefighters, and welders. Patients often complain of:<ref name="Chest1990-ilano">{{cite journal | author=Ilano AL, Raffin TA. | title=Management of carbon monoxide poisoning | journal=Chest | year=1990 | pages=165-9 | volume=97 | issue=1 | id=PMID 2403894}}</ref> | ||
* Persistent [[migraine]] or other [[Headache|headaches]] | |||
* [[Lightheadedness]] | |||
* [[Confusion]] | |||
* [[Nausea]] | |||
*[[flu|Flu-like viral syndromes]] | |||
*[[clinical depression|Depression]] | |||
*[[Chronic fatigue syndrome]] | |||
. Upon removal from exposure, the symptoms usually resolve themselves.<ref name="J Occup Med1999-fawcett">{{cite journal | author=Fawcett TA, Moon RE, Fracica PJ, Mebane GY, Theil DR, Piantadosi CA. | title=Warehouse workers' headache. Carbon monoxide poisoning from propane-fueled forklifts | journal=J Occup Med | year=1992 | pages=12-5 | volume=34 | issue=1 | id=PMID 1552375}}</ref> | |||
==Treatment== | ==Treatment== | ||
''' | === Emergency menagement === | ||
'''Emergency management''' for carbon monoxide poisoning include: | |||
* Immediately remove the victim from the site of exposure, without endangering oneself | |||
* [[Call for help]] | |||
* Start [[Cardiopulmonary resuscitation|CPR]] if needed | |||
Further specific treatment for other complications such as [[seizure]], cardiac | === Medical treatment === | ||
* The primary medical treatment for carbon monoxide poisoning is breathing 100% [[oxygen]] by a tight fitting [[oxygen mask]]. Oxygen hastens the dissociation of carbon monoxide from [[hemoglobin]], improving tissue [[oxygenation]] by reducing its biological half-life. | |||
* [[Hyperbaric oxygen]] is also used in the treatment of [[Carbon monoxide|CO poisoning]]. [[Hyperbaric oxygen]] also increases dissociation and does so to a greater extent than normal [[oxygen]].<ref name="AnnEmergMed1995-thom">{{cite journal | author=Thom SR, Taber RL, Mendiguren II, Clark JM, Hardy KR, Fisher AB. | title=Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen | journal=Ann Emerg Med | year=1995 | pages=474-80 | volume=25 | issue=4 | id=PMID 7710151}}</ref> | |||
* [[Hyperbaric oxygen]] may also facilitate the dissociation of CO from [[Cytochrome c oxidase|cytochrome oxidase.]]<ref name="NEnglJMed2002-weaver">{{cite journal | author=Weaver LK, Hopkins RO, Chan KJ, Churchill S, Elliott CG, Clemmer TP, Orme JF Jr, Thomas FO, Morris AH. | title=Hyperbaric oxygen for acute carbon monoxide poisoning | journal=N Engl J Med | year=2002 | pages=1057-67 | volume=347 | issue=14 | id=PMID 12362006}}</ref> | |||
* There has been a lot of controversy in literature regarding the fact that whether or not [[hyperbaric oxygen]] offers any extra benefits over normal high flow oxygen.<ref name="ToxicolRev2005-buckley" /><ref name="MedJAust1999-Scheinkestel" /><ref name="Lancet1989-raphael">{{cite journal | author=Raphael JC, Elkharrat D, Jars-Guincestre MC, Chastang C, Chasles V, Vercken JB, Gajdos P. | title=Trial of normobaric and hyperbaric oxygen for acute carbon monoxide intoxication | journal=Lancet | year=1989 | pages=414-9 | volume=2 | issue=8660 | id=PMID 2569600}}</ref><ref name="UnderseaHyperbMed1995-ducasse">{{cite journal | author=Ducasse JL, Celsis P, Marc-Vergnes JP. | title=Non-comatose patients with acute carbon monoxide poisoning: hyperbaric or normobaric oxygenation? | journal=Undersea Hyperb Med | year=1995 | pages=9-15 | volume=22 | issue=1 | id=PMID 7742714 | [http://archive.rubicon-foundation.org/2193 pdf]}}</ref> | |||
* Further specific treatment for other complications such as [[seizure]], [[cardiac dysfunction]], [[pulmonary edema]], and [[acidosis]] may be required. | |||
* The delayed development of [[neuropsychiatric]] impairment is one of the most serious complications of CO poisoning, with extensive follow up and treatment often being required. | |||
==Prevention== | ==Prevention== | ||
Prevention | === Primary Prevention === | ||
* Effective measures for primary prevention of CO poisonong include: | |||
The devices, which retail for USD $20-$60 and are widely available, can either be battery-operated or AC powered (with or without a battery backup). Since CO is colorless and odorless (unlike smoke from a fire), detection in a home environment is impossible without such a warning device. Some state and municipal governments, including those of Ontario, Canada, and New York City, require installation of CO detectors in new units. Massachusetts and Illinois began to require a detector in all residences on January 1, 2007. | * Public education on the safe operation of appliances, heaters, fireplaces, and internal-combustion engines | ||
* Installation of [[carbon monoxide detector]]s. | |||
The carbon monoxide can be easily detected by the filtering paper impregnated by the [[solution]] of the [[palladium chloride]]. Carbon monoxide reduces the palladium monoxide to the black metallic [[palladium]]. This reaction is very sensitive. | ** Carbon monoxide alarms are usually installed in homes around heaters and other equipment. | ||
** If a high level of CO is detected, the device sounds an alarm, giving people in the area a chance to ventilate the area or safely leave the building. Unlike [[smoke detector]]s, they do not need to be placed near ceiling level. | |||
** The Consumer Product Safety Commission says that "carbon monoxide detectors are as important to home safety as smoke detectors are," and recommends that each home should have at least one carbon monoxide detector. | |||
** The devices, which retail for USD $20-$60 and are widely available, can either be battery-operated or AC powered (with or without a battery backup). Since CO is colorless and odorless (unlike smoke from a fire), detection in a home environment is impossible without such a warning device. | |||
** Some state and municipal governments, including those of Ontario, Canada, and New York City, require installation of CO detectors in new units. Massachusetts and Illinois began to require a detector in all residences on January 1, 2007. | |||
* The carbon monoxide can be easily detected by the filtering paper impregnated by the [[solution]] of the [[palladium chloride]]. Carbon monoxide reduces the palladium monoxide to the black metallic [[palladium]]. This reaction is very sensitive. | |||
==See also== | ==See also== | ||
*[[Carbon monoxide detector]] | *[[Carbon monoxide detector]] | ||
| Line 180: | Line 210: | ||
* [http://cfpub.epa.gov/airnow/index.cfm?action=where.world International Air Quality] -- Realtime monitors worldwide | * [http://cfpub.epa.gov/airnow/index.cfm?action=where.world International Air Quality] -- Realtime monitors worldwide | ||
* [http://www.epa.gov/air/data/ AirData : Access to Air Pollution Data] -- US EPA annual database | * [http://www.epa.gov/air/data/ AirData : Access to Air Pollution Data] -- US EPA annual database | ||
==References== | ==References== | ||
{{reflist|2}} | |||
[[Category: | [[Category:Pulmonology]] | ||
[[Category: | [[Category:Emergency medicine]] | ||
[[Category:Up-to-Date]] | |||
[[Category:Medicine]] | |||
[[de:Kohlenstoffmonoxidintoxikation]] | [[de:Kohlenstoffmonoxidintoxikation]] | ||
| Line 205: | Line 232: | ||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WS}} | {{WS}} | ||
<references /> | |||
Latest revision as of 13:05, 4 April 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]; Associate Editor(s)-in-Chief: Aditya Govindavarjhulla, M.B.B.S. [4], Fatima Shaukat, MD [5]
Overview
Carbon monoxide poisoning occurs after the inhalation of carbon monoxide gas. Carbon monoxide (CO) is a product of combustion of organic matter under conditions of restricted oxygen supply, which prevents complete oxidation to carbon dioxide (CO2). Carbon monoxide is colorless, odorless, tasteless, and non-irritating, making it difficult to detect. Carbon monoxide is a significantly toxic gas with poisoning being the most common type of fatal poisoning in many countries.[1] Symptoms of mild poisoning include headaches and flu-like effects; larger exposures can lead to significant toxicity of the central nervous system and heart. Following poisoning, long-term sequelae often occur. Carbon monoxide can also have severe effects on the fetus of a pregnant woman. The mechanisms by which carbon monoxide produces toxic effects are not yet fully understood, but hemoglobin, myoglobin, and mitochondrial cytochrome oxidase are thought to be compromised. Treatment largely consists of administering 100% oxygen or hyperbaric oxygen therapy, although the optimum treatment remains controversial.[2] Domestic carbon monoxide poisoning can be prevented by the use of household carbon monoxide detectors.
Historical perspective
- Carbon monoxide poisoning may manifest as lethargy, fatigue and tiredness, depression, memory loss, emotional disturbance, and visual/auditory hallucinations.
- In a few cases, it was associated with 'haunted houses' where the residents experienced strange sounds and visions, feelings of dread, sudden onset illness, and death of all the members in the house.
- In 1921, Dr. William Wilmer, an ophthalmologist published a case in American journal of ophthalmology in which he described the experiences of a family with similar symptoms as mentioned above.[3]
- In 2005, a report was published describing a young female of age 23, found delirious and hyperventilating, as a result of leakage and accumulation of gas from the heater. She believed to have seen a 'ghost' in the shower. [4]
Sources
- Main sources of CO that are responsible for environmental pollution are house hold fires, heaters, furnaces, motor vehicle exhaust, propane fueled equipment (such as ice resurfacers, forklifts), engine-driven generators, and wood burning stove.[5][6]
- CO poisoning can also occur in scuba diving due to malfunctioning of diving air compressors.
- Another source is exposure to the organic solvent methylene chloride, which is metabolized to CO by the body.[7]
- Polluted air often contains unhealthy levels of carbon monoxide. Many areas of the US have struggled to achieve legislated limits. Significant advances have been made since the implementation by 1990 of a vehicle emissions limit of 3.4 gpm (grams per mile), a large reduction from the previous limit of 87 gpm.[8]
Pathophysiology
The precise mechanisms by which toxic effects are induced by CO are not fully understood. It may damage the body in one of the following ways:[9]
- Binds to hemoglobin thus reducing oxygen transportation in blood[10]
- Binds with myoglobin to decrease its oxygen storage capacity[11]
- Reacts with mitochondrial cytochrome oxidase (specifically cytochrome C) hence inhibiting cellular respiration.[12]
CO and Hemoglobin
- Carbon monoxide (CO) has a significant affinity to the iron sites in hemoglobin, the principal oxygen-carrying compound in blood. The affinity between carbon monoxide and hemoglobin is 240 times stronger than the affinity between hemoglobin and oxygen.
- CO binds to hemoglobin, producing carboxyhemoglobin (COHb) - the traditional belief is that carbon monoxide toxicity arises from the formation of carboxyhemoglobin, which decreases the oxygen-carrying capacity of the blood. This inhibits the transport, delivery, and utilization of oxygen.[13]
- Because hemoglobin is a tetramer with four oxygen binding sites, binding of CO at one of these sites also increases the oxygen affinity of the remaining 3 sites, which interferes with normal release of oxygen. This causes hemoglobin to retain oxygen that would otherwise be delivered to the tissue.[14]
- Amount of oxygen available for tissue use are decreased. This situation is described as CO shifting the oxygen dissociation curve to the left. Blood oxygen content is actually increased in the case of carbon monoxide poisoning; because all the oxygen is in the blood, none is being given to the tissues, and this causes tissue hypoxic injury.
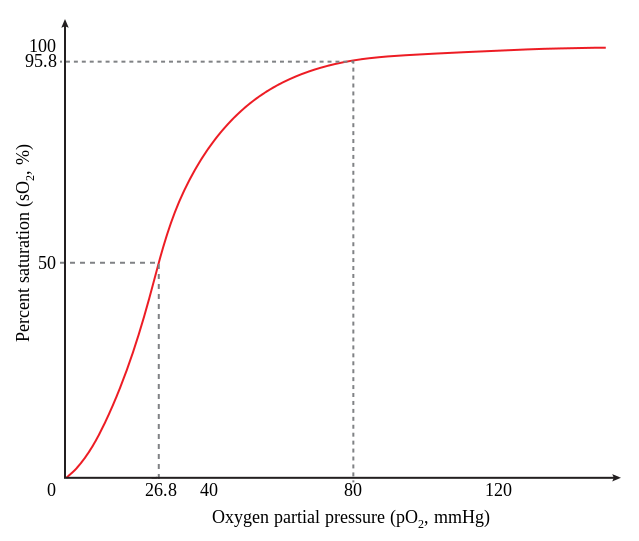
CO and Myoglobin
- CO has 60 times greater affinity to myoglobin as compared to oxygen. It can bind readily to myoglobin, which is another heme-containing protein, abundantly present in the muscles of body.
- Binding of CO to myoglobin markedly reduces its oxygen carrying capacity, leading to decrease in cardiac output, hypotension which ultimately resulting in cerebral ischemia.
- CO bound to myolobin is later released in the body, which subsequently binds with the hemoglobin. This phenomenon is responsible for delayed return of symptoms in CO poisoning. [15]
CO and Mitochondrial Cytochrome Oxidase C
- CO has lesser affinity with cytochrome c oxidase in mitochondria than oxygen so the damage occurs only in the setting of significant hypoxia.
- CO damages the cell by interfering with aerobic metabolism and synthesis of ATP, which leads to shifting of metabolism to anaerobic and accumulation of lactic acid intracellularly with significant anoxia. This ultimately leads to necrosis and cell death.
- Once CO is bound to cytochrome c oxidase, the rate of dissociation is very slow. Hence, causing prolonged damage to oxidaive metabolism in the cell.[16]
Epidemiology & Demographics
- Carbon monoxide poisoning is the most common type of fatal poisoning in France and the United States. It has been estimated that more than 40,000 people per year seek medical attention for carbon monoxide poisoning in the United States.[17]
- In many industrialized countries, carbon monoxide may be the cause of greater than 50% of fatal poisonings.
- In the U.S., about 200 people die each year from carbon monoxide poisoning associated with home fuel-burning heating equipment. The CDC reports, "Each year, more than 500 Americans die from unintentional CO poisoning, and more than 2,000 commit suicide by intentionally poisoning themselves."[18]
CO and Suicide
- Carbon monoxide has emerged as a common mean of suicide by poisoning, since the placement of strict legal restrictions on poisons like cyanide and arsenic.
- Suicide was also often committed by inhaling exhaust fumes of running car engines. In the past, motor car exhaust may have contained up to 25% carbon monoxide. However, newer cars have catalytic converters, which can eliminate over 99% of carbon monoxide produced.[19] However, even cars with catalytic converters can produce substantial carbon monoxide if an idling car is left in an enclosed space. This is due to reduced oxygen availability, and therefore, less efficient combustion.
- As carbon monoxide poisoning via car exhaust has become less of a suicide option, there has been an increase in new methods of carbon monoxide poisoning such as burning charcoal or other fossil fuels within a confined space, such as a small room, tent, or car.[20]
Toxicity
- Carbon monoxide is a significantly toxic gas, although patients may demonstrate varied clinical manifestations with different outcomes, even under similar exposure conditions.[21]
Risk factors
- Toxicity is also increased by several factors as follows:
- Increased activity and rate of ventilation
- Pre-existing cerebral or cardiovascular disease
- Reduced cardiac output
- Anemia or other hematological disorders
- Decreased barometric pressure
- High metabolic rate.
Classification:
- The degrees of poisoning have been described as mild, moderate, and severe based on carboxyhaemoglobin percentage levels and clinical symptoms.:[22]
- Mild carbon monoxide poisoning: Carboxyhaemoglobin level of over 10% without clinical signs or symptoms of carbon monoxide poisoning.
- Moderate carbon monoxide poisoning: Carboxyhaemoglobin level of over 10%, but under 20-25%, with minor clinical signs and symptoms of poisoning, such as headache, lethargy, or fatigue.
- Severe carbon monoxide poisoning: Carboxyhaemoglobin level of over 20-25%, loss of consciousness, and confusion or signs of cardiac ischemia, or both.
- Levels of carbon monoxide bound in the blood can be determined by measuring carboxyhaemoglobin, which is a stable complex of carbon monoxide and hemoglobin that forms in red blood cells.
- Carbon monoxide also functions as a neurotransmitter.
- Normal carboxyhemoglobin levels in an average person are less than 5%, whereas cigarette smokers (two packs/day) may have levels up to 9%.[23]
- Serious toxicity is often associated with carboxyhemoglobin levels above 25%, and the risk of fatality is high with levels over 70%. Still, no consistent dose response relationship has been found between carboxyhemoglobin levels and clinical effects.[24]
- Hence, carboxyhemoglobin levels are better guide for exposure levels than effects as they do not reliably predict clinical course or short- or long-term outcome.[25]
- The effects of carbon monoxide in parts per million are listed below:[26]
- 35 ppm (0.0035%): Headache and dizziness within six to eight hours of constant exposure.
- 100 ppm (0.01%): Slight headache in two to three hours.
- 200 ppm (0.02%): Slight headache within two to three hours.
- 400 ppm (0.04%): Frontal headache within one to two hours.
- 800 ppm (0.08%): Dizziness, nausea, and convulsions within 45 minutes. Insensible within two hours.
- 1,600 ppm (0.16%): Headache, dizziness, and nausea within 20 minutes. Death in less than two hours.
- 3,200 ppm (0.32%): Headache, dizziness and nausea in five to ten minutes. Death within 30 minutes.
- 6,400 ppm (0.64%): Headache and dizziness in one to two minutes. Death in less than 20 minutes.
- 12,800 ppm (1.28%): Unconsciousness after 2-3 breaths. Death in less than three minutes.
- In addition, a recent report concludes that carbon monoxide exposure can lead to a decrease in lifespan significantly as a result of damaged myocardium.[27]
Signs and Symptoms
Carbon monoxide poisoning is particularly difficult to diagnose since it is not easily detected by human senses. Since CO poisoning is a diagnosis frequently overlooked, it is overly emphasized to measure carboxyhemoglobin in suspicious cases. The clinical manifestations include :
Acute
Most common:
Common symptoms of acute CO poisoning include:[28]
Less common
Less common symptoms of acute CO poisoning are as follows:[29]
- Myocardial ischemia
- Atrial fibrillation
- Pneumonia
- Pulmonary edema
- Hyperglycemia
- Lactic acidosis
- Muscle necrosis
- Acute renal failure
- Skin lesions
- Visual and auditory dysfunction
Delayed
CO poisoning may cause delayed clinical manifestations in the form of severe neurological manifestations.These symptoms occur days or even weeks after an acute poisoning epispde. Common clinical manifestations include:[30][31]
- Difficulty with higher intellectual functions and short-term memory
Chronic
Long term, repeat exposures present a greater risk to individuals with coronary heart disease and in pregnant patients.[32] Chronic exposure may increase the incidence of cardiovascular symptoms in some workers, such as motor vehicle examiners, firefighters, and welders. Patients often complain of:[33]
- Persistent migraine or other headaches
- Lightheadedness
- Confusion
- Nausea
. Upon removal from exposure, the symptoms usually resolve themselves.[34]
Treatment
Emergency menagement
Emergency management for carbon monoxide poisoning include:
- Immediately remove the victim from the site of exposure, without endangering oneself
- Call for help
- Start CPR if needed
Medical treatment
- The primary medical treatment for carbon monoxide poisoning is breathing 100% oxygen by a tight fitting oxygen mask. Oxygen hastens the dissociation of carbon monoxide from hemoglobin, improving tissue oxygenation by reducing its biological half-life.
- Hyperbaric oxygen is also used in the treatment of CO poisoning. Hyperbaric oxygen also increases dissociation and does so to a greater extent than normal oxygen.[35]
- Hyperbaric oxygen may also facilitate the dissociation of CO from cytochrome oxidase.[36]
- There has been a lot of controversy in literature regarding the fact that whether or not hyperbaric oxygen offers any extra benefits over normal high flow oxygen.[2][25][37][38]
- Further specific treatment for other complications such as seizure, cardiac dysfunction, pulmonary edema, and acidosis may be required.
- The delayed development of neuropsychiatric impairment is one of the most serious complications of CO poisoning, with extensive follow up and treatment often being required.
Prevention
Primary Prevention
- Effective measures for primary prevention of CO poisonong include:
- Public education on the safe operation of appliances, heaters, fireplaces, and internal-combustion engines
- Installation of carbon monoxide detectors.
- Carbon monoxide alarms are usually installed in homes around heaters and other equipment.
- If a high level of CO is detected, the device sounds an alarm, giving people in the area a chance to ventilate the area or safely leave the building. Unlike smoke detectors, they do not need to be placed near ceiling level.
- The Consumer Product Safety Commission says that "carbon monoxide detectors are as important to home safety as smoke detectors are," and recommends that each home should have at least one carbon monoxide detector.
- The devices, which retail for USD $20-$60 and are widely available, can either be battery-operated or AC powered (with or without a battery backup). Since CO is colorless and odorless (unlike smoke from a fire), detection in a home environment is impossible without such a warning device.
- Some state and municipal governments, including those of Ontario, Canada, and New York City, require installation of CO detectors in new units. Massachusetts and Illinois began to require a detector in all residences on January 1, 2007.
- The carbon monoxide can be easily detected by the filtering paper impregnated by the solution of the palladium chloride. Carbon monoxide reduces the palladium monoxide to the black metallic palladium. This reaction is very sensitive.
See also
- Carbon monoxide detector
- Carbon Dioxide Poisoning
- List of deaths from carbon monoxide poisoning
- Undersea and Hyperbaric Medical Society
Resources
- Carbonmonoxidekills.com: Carbon Monoxide Poisoning Protection Video
- Carbonmonoxide.net: Carbon Monoxide Poisoning Support Forum
- COALERT: Carbon monoxide poisoning information, co detectors, co detector placement
- 2003 report of a group suicide via charcoal-produced carbon monoxide poisoning, in Japan
- 2005 report of a group suicide via charcoal-produced carbon monoxide poisoning, in the UK
- COSUPPORT: Carbon monoxide study from UK carbon monoxide poisoning victims support group
- International Air Quality -- Realtime monitors worldwide
- AirData : Access to Air Pollution Data -- US EPA annual database
References
- ↑ Omaye ST. (2002). "Metabolic modulation of carbon monoxide toxicity". Toxicology. 180 (2): 139–50. PMID 12324190.
- ↑ 2.0 2.1 Buckley NA, Isbister GK, Stokes B, Juurlink DN. (2005). "Hyperbaric oxygen for carbon monoxide poisoning : a systematic review and critical analysis of the evidence". Toxicol Rev. 24 (2): 75–92. PMID 16180928.
- ↑ [1]
- ↑ Jiann-Ruey Ong, Sheng-Wen Hou, Hsien-Tsung Shu, Huei-Tsair Chen, and Chee-Fah Chong. Diagnostic pitfall: carbon monoxide poisoning mimicking hyperventilation syndrome. The American Journal of Emergency Medicine Volume 23, Issue 7 , November 2005, Pages 903-904
- ↑ Johnson C, Moran J, Paine S, Anderson H, Breysse P (1975). "Abatement of toxic levels of carbon monoxide in Seattle ice-skating rinks". Am J Public Health. 65 (10): 1087–90. PMID 1163706.
- ↑ Fawcett T, Moon R, Fracica P, Mebane G, Theil D, Piantadosi C (1992). "Warehouse workers' headache. Carbon monoxide poisoning from propane-fueled forklifts". J Occup Med. 34 (1): 12–5. PMID 1552375.
- ↑ Kubic VL, Anders MW. (1975). "Metabolism of dihalomethanes to carbon monoxide. II. In vitro studies". Drug Metab Dispos. 3 (2): 104–12. PMID 236156.
- ↑ "Carbon Monoxide (CO) Pollution in Outdoor Air | US EPA".
- ↑ Brunori M, Bonaventura J, Bonaventura C, Antonini E, Wyman J (1972). "Carbon monoxide binding by hemoglobin and myoglobin under photodissociating conditions". Proc Natl Acad Sci U S A. 69 (4): 868–71. PMC 426583. PMID 4502938.
- ↑ Culnan DM, Craft-Coffman B, Bitz GH, Capek KD, Tu Y, Lineaweaver WC; et al. (2018). "Carbon Monoxide and Cyanide Poisoning in the Burned Pregnant Patient: An Indication for Hyperbaric Oxygen Therapy". Ann Plast Surg. doi:10.1097/SAP.0000000000001351. PMID 29461288.
- ↑ Lim M, Jackson TA, Anfinrud PA (1995). "Binding of CO to myoglobin from a heme pocket docking site to form nearly linear Fe-C-O". Science. 269 (5226): 962–6. PMID 7638619.
- ↑ Alonso JR, Cardellach F, López S, Casademont J, Miró O (2003). "Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain". Pharmacol Toxicol. 93 (3): 142–6. PMID 12969439.
- ↑ Haldane J. (1895). "The action of carbonic oxide on man". J Physiol. 18: 430–62.
- ↑ Gorman D, Drewry A, Huang YL, Sames C. (2003). "The clinical toxicology of carbon monoxide". Toxicology. 187 (1): 25–38. PMID 12679050.
- ↑ Omaye ST (2002). "Metabolic modulation of carbon monoxide toxicity". Toxicology. 180 (2): 139–50. PMID 12324190.
- ↑ Blumenthal I (2001). "Carbon monoxide poisoning". J R Soc Med. 94 (6): 270–2. PMC 1281520. PMID 11387414.
- ↑ Hampson NB. (1998). "Emergency department visits for carbon monoxide poisoning in the Pacific Northwest". J Emerg Med. 16 (5): 695–8. PMID 9752939.
- ↑ [2]
- ↑ Vossberg B, Skolnick J. (1999). "The role of catalytic converters in automobile carbon monoxide poisoning: a case report". Chest. 115 (2): 580–1. PMID 10027464.
- ↑ Chung WS, Leung CM. (2001). "Carbon monoxide poisoning as a new method of suicide in Hong Kong". Psychiatr Serv. 52 (6): 836–7. PMID 11376237.
- ↑ Raub JA, Mathieu-Nolf M, Hampson NB, Thom SR. (2000). "Carbon monoxide poisoning-a public health perspective". Toxicology. 145 (1): 1–14. PMID 10771127.
- ↑ Olson K, Smollin C (2008). "Carbon monoxide poisoning (acute)". BMJ Clin Evid. 2008. PMC 2907971. PMID 19445736.
- ↑ Ford MD, Delaney KA, Ling LJ, Erickson T., ed. (2001). Clinical toxicology. WB Saunders Company. ISBN 0-7216-5485-1.
- ↑ Hardy KR, Thom SR. (1994). "Pathophysiology and treatment of carbon monoxide poisoning". J Toxicol Clin Toxicol. 32 (6): 613–29. PMID 7966524.
- ↑ 25.0 25.1 Scheinkestel CD, Bailey M, Myles PS, Jones K, Cooper DJ, Millar IL, Tuxen DV. (1999). "Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: a randomised controlled clinical trial". Med J Aust. 170 (5): 203–10. PMID 10092916.
- ↑ Goldstein M (2008). "Carbon monoxide poisoning". J Emerg Nurs. 34 (6): 538–42. doi:10.1016/j.jen.2007.11.014. PMID 19022078.
- ↑ Henry CR, Satran D, Lindgren B, Adkinson C, Nicholson CI, Henry TD, MD (2006). "Myocardial Injury and Long-term Mortality Following Moderate to Severe Carbon Monoxide Poisoning". JAMA. 295: 398–402. Abstract
- ↑ Quinn DK, McGahee SM, Politte LC, Duncan GN, Cusin C, Hopwood CJ; et al. (2009). "Complications of carbon monoxide poisoning: a case discussion and review of the literature". Prim Care Companion J Clin Psychiatry. 11 (2): 74–9. PMC 2707118. PMID 19617936.
- ↑ Choi IS. (2001). "Carbon monoxide poisoning: systemic manifestations and complications". J Korean Med Sci. 16 (3): 253–61. PMID 11410684.
- ↑ Roohi F, Kula RW, Mehta N. (2001). "Twenty-nine years after carbon monoxide intoxication". Clin Neurol Neurosurg. 103 (2): 92–5. PMID 11516551.
- ↑ Choi IS (2001). "Carbon monoxide poisoning: systemic manifestations and complications". J Korean Med Sci. 16 (3): 253–61. doi:10.3346/jkms.2001.16.3.253. PMC 3054741. PMID 11410684.
- ↑ Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J. (1989). "Short-term effects of carbon monoxide exposure on the exercise performance of subjects with coronary artery disease". N Engl J Med. 321 (21): 1426–32. PMID 2682242.
- ↑ Ilano AL, Raffin TA. (1990). "Management of carbon monoxide poisoning". Chest. 97 (1): 165–9. PMID 2403894.
- ↑ Fawcett TA, Moon RE, Fracica PJ, Mebane GY, Theil DR, Piantadosi CA. (1992). "Warehouse workers' headache. Carbon monoxide poisoning from propane-fueled forklifts". J Occup Med. 34 (1): 12–5. PMID 1552375.
- ↑ Thom SR, Taber RL, Mendiguren II, Clark JM, Hardy KR, Fisher AB. (1995). "Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen". Ann Emerg Med. 25 (4): 474–80. PMID 7710151.
- ↑ Weaver LK, Hopkins RO, Chan KJ, Churchill S, Elliott CG, Clemmer TP, Orme JF Jr, Thomas FO, Morris AH. (2002). "Hyperbaric oxygen for acute carbon monoxide poisoning". N Engl J Med. 347 (14): 1057–67. PMID 12362006.
- ↑ Raphael JC, Elkharrat D, Jars-Guincestre MC, Chastang C, Chasles V, Vercken JB, Gajdos P. (1989). "Trial of normobaric and hyperbaric oxygen for acute carbon monoxide intoxication". Lancet. 2 (8660): 414–9. PMID 2569600.
- ↑ Ducasse JL, Celsis P, Marc-Vergnes JP. (1995). "Non-comatose patients with acute carbon monoxide poisoning: hyperbaric or normobaric oxygenation?". Undersea Hyperb Med. 22 (1): 9–15. PMID 7742714. Text " pdf" ignored (help)
de:Kohlenstoffmonoxidintoxikation it:Monossido di carbonio#Tossicit.C3.A0 nl:Koolstofmonoxide#Vergiftiging no:Kullosforgiftning fi:Häkämyrkytys sv:Kolmonoxidförgiftning