Amlodipine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Amlodipine is a calcium channel blocker, dihydropirydine calcium channel blocker that is FDA approved for the treatment of hypertension, coronary artery disease. Common adverse reactions include flushing, palpitations, peripheral edema, abdominal pain, nausea, dizziness, headache, somnolence, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension
- Dosing Information
- The usual initial antihypertensive oral dose of amlodipine is 5 mg once daily with a maximum dose of 10 mg once daily.
Small, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 2.5 mg once daily and this dose may be used when adding amlodipine to other antihypertensive therapy.
Adjust dosage according to each patient's need. In general, titration should proceed over 7 to 14 days so that the physician can fully assess the patient's response to each dose level. Titration may proceed more rapidly, however, if clinically warranted, provided the patient is assessed frequently.
Coronary Artery Disease
- Dosing Information
- The recommended dose for chronic stable or vasospastic angina is 5–10 mg, with the lower dose suggested in the elderly and in patients with hepatic insufficiency. Most patients will require 10 mg for adequate effect [see Adverse Reactions].
The recommended dose range for patients with coronary artery disease is 5–10 mg once daily. In clinical studies, the majority of patients required 10 mg
Off-Label Use and Dosage (Adult)
Non–Guideline-Supported Use
Diabetic Nephropathy
- Dosing Information
Nondiabetic Kidney Disease
- Dosing Information
- 5-10 mg/day.[3]
Left Ventricular Hypertrophy
- Dosing Information
- Monotherapy: 5 mg/day, increase to 10 mg/day after first 14 days of treatment.
- Combination therapy: Amlodipine 5 mg/day for 14 days, then amlodipine 5 mg/day + benazepril 10 mg/day.[4]
Raynaud's Phenomenon
- Dosing Information
- 10 mg PO q24h.[5]
Silent Myocardial Ischemia
- Dosing Information
Systolic Hypertension
- Dosing Information
- 5 mg PO q24h.[9]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Hypertension
- Dosing Information
- The effective antihypertensive oral dose in pediatric patients ages 6–17 years is 2.5 mg to 5 mg once daily. Doses in excess of 5 mg daily have not been studied in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Contraindications
- Condition 1
- Condition 2
- Condition 3
- Condition 4
- Condition 5
Warnings
Conidition 1
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
| |
Amlodipine
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
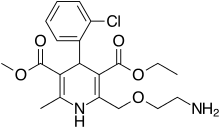
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Amlodipine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Amlodipine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Amlodipine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Amlodipine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Amlodipine Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Seccia, T.M.; Vulpis, V.; Ricci, S.; Pirrelli, A. (1995). "Antihypertensive and Metabolic Effects of Amlodipine in Patients with Non-Insulin-Dependent Diabetes Mellitus". Clinical Drug Investigation. 9 (1): 16–21. doi:10.2165/00044011-199509010-00004. ISSN 1173-2563.
- ↑ Fogari R, Preti P, Zoppi A, Rinaldi A, Corradi L, Pasotti C; et al. (2002). "Effects of amlodipine fosinopril combination on microalbuminuria in hypertensive type 2 diabetic patients". Am J Hypertens. 15 (12): 1042–9. PMID 12460699.
- ↑ Esnault VL, Brown EA, Apetrei E, Bagon J, Calvo C, DeChatel R; et al. (2008). "The effects of amlodipine and enalapril on renal function in adults with hypertension and nondiabetic nephropathies: a 3-year, randomized, multicenter, double-blind, placebo-controlled study". Clin Ther. 30 (3): 482–98. doi:10.1016/j.clinthera.2008.03.006. PMID 18405787.
- ↑ Neutel JM, Smith DH, Weber MA (2004). "Effect of antihypertensive monotherapy and combination therapy on arterial distensibility and left ventricular mass". Am J Hypertens. 17 (1): 37–42. PMID 14700510.
- ↑ La Civita L, Pitaro N, Rossi M, Gambini I, Giuggioli D, Cini G; et al. (1993). "Amlodipine in the treatment of Raynaud's phenomenon". Br J Rheumatol. 32 (6): 524–5. PMID 8508292.
- ↑ Deanfield JE, Detry JM, Lichtlen PR, Magnani B, Sellier P, Thaulow E (1994). "Amlodipine reduces transient myocardial ischemia in patients with coronary artery disease: double-blind Circadian Anti-Ischemia Program in Europe (CAPE Trial)". J Am Coll Cardiol. 24 (6): 1460–7. PMID 7930276.
- ↑ Madjlessi-Simon T, Fillette F, Mary-Krause M, Lechat P, Jaillon P (1995). "Effects of amlodipine on transient myocardial ischaemia in patients with a severe coronary condition treated with a beta-blocker. Amlor-Holter Study Investigators". Eur Heart J. 16 (12): 1780–8. PMID 8682007.
- ↑ Bech J, Madsen JK, Kelbaek H (1999). "Amlodipine reduces myocardial ischaemia during exercise without compromising left ventricular function in patients with silent ischaemia: a randomised, double-blind, placebo-controlled study". Eur J Heart Fail. 1 (4): 395–400. PMID 10937953.
- ↑ Malacco E, Varì N, Capuano V, Spagnuolo V, Borgnino C, Palatini P; et al. (2003). "A randomized, double-blind, active-controlled, parallel-group comparison of valsartan and amlodipine in the treatment of isolated systolic hypertension in elderly patients: the Val-Syst study". Clin Ther. 25 (11): 2765–80. PMID 14693303.