Adrenocortical carcinoma pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
==Pathophysiology== | ==Pathophysiology== | ||
ACCs are typically large tumors upon clinical presentation, often measuring more than 6 cm in diameter. <ref name="pmid19755599">{{cite journal| author=Johnson PT, Horton KM, Fishman EK| title=Adrenal mass imaging with multidetector CT: pathologic conditions, pearls, and pitfalls. | journal=Radiographics | year= 2009 | volume= 29 | issue= 5 | pages= 1333-51 | pmid=19755599 | doi=10.1148/rg.295095027 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19755599 }} </ref> | * ACCs are typically large tumors upon clinical presentation, often measuring more than 6 cm in diameter.<ref name="pmid19755599">{{cite journal| author=Johnson PT, Horton KM, Fishman EK| title=Adrenal mass imaging with multidetector CT: pathologic conditions, pearls, and pitfalls. | journal=Radiographics | year= 2009 | volume= 29 | issue= 5 | pages= 1333-51 | pmid=19755599 | doi=10.1148/rg.295095027 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19755599 }} </ref> | ||
* They are bilateral in 2% to 10% of cases. | |||
They are bilateral in 2% to 10% of cases | * Metastases to the liver, lungs, or lymph nodes can be seen, and invasion of adjacent organs or venous extension into the renal vein and/or inferior vena cava may be present.<ref name="pmid7142516">{{cite journal| author=Dunnick NR, Heaston D, Halvorsen R, Moore AV, Korobkin M| title=CT appearance of adrenal cortical carcinoma. | journal=J Comput Assist Tomogr | year= 1982 | volume= 6 | issue= 5 | pages= 978-82 | pmid=7142516 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7142516 }}</ref> | ||
* Inferior vena cava invasion has been reported in 9% to 19% of cases at presentation.<ref name="pmid21606258">{{cite journal| author=Bharwani N, Rockall AG, Sahdev A, Gueorguiev M, Drake W, Grossman AB et al.| title=Adrenocortical carcinoma: the range of appearances on CT and MRI. | journal=AJR Am J Roentgenol | year= 2011 | volume= 196 | issue= 6 | pages= W706-14 | pmid=21606258 | doi=10.2214/AJR.10.5540 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21606258 }}</ref> | |||
Metastases to the liver, lungs, or lymph nodes can be seen, and invasion of adjacent organs or venous extension into the renal vein and/or inferior vena cava may be present. | * Due to the presence of internal hemorrhage, necrosis, and calcifications, these tumors tend to vary in appearance with frequent heterogeneous enhancement. | ||
Spread can take several forms:<ref name="pmid19326954">{{cite journal| author=Dehner LP, Hill DA| title=Adrenal cortical neoplasms in children: why so many carcinomas and yet so many survivors? | journal=Pediatr Dev Pathol | year= 2009 | volume= 12 | issue= 4 | pages= 284-91 | pmid=19326954 | doi=10.2350/08-06-0489.1 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19326954 }}</ref> | |||
Inferior vena cava invasion has been reported in 9% to 19% of cases at presentation | |||
Due to the presence of internal hemorrhage, necrosis, and calcifications, these tumors tend to vary in appearance with frequent heterogeneous enhancement. | |||
Spread can take several forms: | |||
* Direct invasion of the tumor capsule, invasion through the tumor capsule into extra-adrenal soft tissue | * Direct invasion of the tumor capsule, invasion through the tumor capsule into extra-adrenal soft tissue | ||
* Direct invasion of lymphatic vessels in and around the capsule and near blood vessels. | * Direct invasion of lymphatic vessels in and around the capsule and near blood vessels. | ||
* Metastatic deposits are largely similar to the primary tumor | * Metastatic deposits are largely similar to the primary tumor | ||
ACCs can be graded into low- and high-grade carcinoma groups based on their mitotic rates ( >20 mitoses per 50 high-power fields vs <20 mitoses per 50 high-power fields) | ACCs can be graded into low- and high-grade carcinoma groups based on their mitotic rates ( >20 mitoses per 50 high-power fields vs. <20 mitoses per 50 high-power fields) | ||
Mitotic rate was most closely associated with patient outcome | Mitotic rate was most closely associated with patient outcome. | ||
ACCs in children behave in a more indolent fashion compared with adult, that is why there are so many pediatric ACCs but few pediatric deaths. | ACCs in children behave in a more indolent fashion compared with the adult, that is why there are so many pediatric ACCs but few pediatric deaths.<ref name="pmid3697922">{{cite journal| author=Cagle PT, Hough AJ, Pysher TJ, Page DL, Johnson EH, Kirkland RT et al.| title=Comparison of adrenal cortical tumors in children and adults. | journal=Cancer | year= 1986 | volume= 57 | issue= 11 | pages= 2235-7 | pmid=3697922 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3697922 }}</ref> | ||
==Genetics== | ==Genetics== | ||
| Line 31: | Line 26: | ||
'''''1. [[Clone (cell biology)|Clonality]]''''' | '''''1. [[Clone (cell biology)|Clonality]]''''' | ||
* ACCs initiate from [[Monoclonal|monoclonal cell]] populations, suggesting that [[mutation]] events lead to [[Clonal selection|clonal expansion]] and ultimate progression to [[cancer]]. | * ACCs initiate from [[Monoclonal|monoclonal cell]] populations, suggesting that [[mutation]] events lead to [[Clonal selection|clonal expansion]] and ultimate progression to [[cancer]].<ref name="pmid7915195">{{cite journal| author=Beuschlein F, Reincke M, Karl M, Travis WD, Jaursch-Hancke C, Abdelhamid S et al.| title=Clonal composition of human adrenocortical neoplasms. | journal=Cancer Res | year= 1994 | volume= 54 | issue= 18 | pages= 4927-32 | pmid=7915195 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7915195 }}</ref> | ||
* [[Flow cytometry]] revealed [[aneuploidy]] in ACC | * [[Flow cytometry]] revealed [[aneuploidy]] in ACC. [[aneuploidy]] was observed in 75% of ACC.<ref name="pmid7910530">{{cite journal| author=Gicquel C, Leblond-Francillard M, Bertagna X, Louvel A, Chapuis Y, Luton JP et al.| title=Clonal analysis of human adrenocortical carcinomas and secreting adenomas. | journal=Clin Endocrinol (Oxf) | year= 1994 | volume= 40 | issue= 4 | pages= 465-77 | pmid=7910530 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7910530 }}</ref> | ||
* Assessment of [[aneuploidy]] with [[histopathological]] criteria in 7 of 9 [[Adrenal tumor|adrenal tumors]] revealed a high correlation with Weiss score >3 (indicative of [[malignancy]]) | * Assessment of [[aneuploidy]] with [[histopathological]] criteria in 7 of 9 [[Adrenal tumor|adrenal tumors]] revealed a high correlation with Weiss score >3 (indicative of [[malignancy]]).<ref name="pmid3617290">{{cite journal| author=Amberson JB, Vaughan ED, Gray GF, Naus GJ| title=Flow cytometric determination of nuclear DNA content in benign adrenal pheochromocytomas. | journal=Urology | year= 1987 | volume= 30 | issue= 2 | pages= 102-4 | pmid=3617290 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3617290 }}</ref> | ||
* No significant difference in overall survival was observed in patients with ACC exhibiting [[aneuploidy]] vs patients with ACC exhibiting [[Diploids|diploid]] [[Neoplasm|neoplasms]]. | * No significant difference in overall survival was observed in patients with ACC exhibiting [[aneuploidy]] vs patients with ACC exhibiting [[Diploids|diploid]] [[Neoplasm|neoplasms]].<ref name="pmid2403197">{{cite journal| author=Cibas ES, Medeiros LJ, Weinberg DS, Gelb AB, Weiss LM| title=Cellular DNA profiles of benign and malignant adrenocortical tumors. | journal=Am J Surg Pathol | year= 1990 | volume= 14 | issue= 10 | pages= 948-55 | pmid=2403197 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2403197 }}</ref> | ||
'''''2. [[Gene expression]] [[DNA microarray|arrays]]''''' | '''''2. [[Gene expression]] [[DNA microarray|arrays]]''''' | ||
* An initial study identified elevated [[Gene expression|expression of genes]] involved in cell proliferation in ACC, such as ''[[IGF2]]'', compared with increased [[Gene expression|expression]] of steroidogenic [[genes]] in ACA. | * An initial study identified elevated [[Gene expression|expression of genes]] involved in cell proliferation in ACC, such as ''[[IGF2]]'', compared with increased [[Gene expression|expression]] of steroidogenic [[genes]] in ACA.<ref name="pmid15613424">{{cite journal| author=de Fraipont F, El Atifi M, Cherradi N, Le Moigne G, Defaye G, Houlgatte R et al.| title=Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic Acid microarrays identifies several candidate genes as markers of malignancy. | journal=J Clin Endocrinol Metab | year= 2005 | volume= 90 | issue= 3 | pages= 1819-29 | pmid=15613424 | doi=10.1210/jc.2004-1075 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15613424 }}</ref> | ||
* Giordano et al identified unique [[Transcription (genetics)|transcriptionally]] activated (12q and 5q) and repressed (11q, 1p, and 17p) [[chromosomal]] regions in 33 ACCs vs 22 ACAs in a [[DNA microarray|microarray]] study. | * Giordano et al identified unique [[Transcription (genetics)|transcriptionally]] activated (12q and 5q) and repressed (11q, 1p, and 17p) [[chromosomal]] regions in 33 ACCs vs 22 ACAs in a [[DNA microarray|microarray]] study.<ref name="pmid19147773">{{cite journal| author=Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J et al.| title=Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. | journal=Clin Cancer Res | year= 2009 | volume= 15 | issue= 2 | pages= 668-76 | pmid=19147773 | doi=10.1158/1078-0432.CCR-08-1067 | pmc=2629378 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19147773 }}</ref> | ||
* Giordano et al (192) determined that ACC with high [[histological]] [[Grading (tumors)|grade]] exhibited overexpression of [[cell cycle]] and functional [[aneuploidy]] [[genes]] and leading to the decreased survival of patients. | * Giordano et al (192) determined that ACC with high [[histological]] [[Grading (tumors)|grade]] exhibited overexpression of [[cell cycle]] and functional [[aneuploidy]] [[genes]] and leading to the decreased survival of patients. | ||
* Expression levels of ''BUB1B,'' ''[[PINK1]], and [[DLG7]]'' ''are'' identified in ACC. | * Expression levels of ''BUB1B,'' ''[[PINK1]], and [[DLG7]]'' ''are'' identified in ACC.<ref name="pmid19139432">{{cite journal| author=de Reyniès A, Assié G, Rickman DS, Tissier F, Groussin L, René-Corail F et al.| title=Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. | journal=J Clin Oncol | year= 2009 | volume= 27 | issue= 7 | pages= 1108-15 | pmid=19139432 | doi=10.1200/JCO.2008.18.5678 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19139432 }}</ref> | ||
==== 3. '''''[[MicroRNAs]]''''' ==== | ==== 3. '''''[[MicroRNAs]]''''' ==== | ||
* [[MicroRNAs]] are [[RNA|RNAs]] that are important in the regulation of [[gene expression]]. | * [[MicroRNAs]] are [[RNA|RNAs]] that are important in the regulation of [[gene expression]]. | ||
* Numerous [[MicroRNA|miRNAs]] have been identified in the regulation of various [[cellular]] processes such as [[proliferation]], [[Apoptosis|apoptosis,]] and [[differentiation]]. | * Numerous [[MicroRNA|miRNAs]] have been identified in the regulation of various [[cellular]] processes such as [[proliferation]], [[Apoptosis|apoptosis,]] and [[differentiation]].<ref name="pmid21116305">{{cite journal| author=Czech B, Hannon GJ| title=Small RNA sorting: matchmaking for Argonautes. | journal=Nat Rev Genet | year= 2011 | volume= 12 | issue= 1 | pages= 19-31 | pmid=21116305 | doi=10.1038/nrg2916 | pmc=3703915 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21116305 }}</ref> | ||
* Dysregulation of miRNAs, such as overexpression or deletion, plays an important role in diseases. | * Dysregulation of miRNAs, such as overexpression or deletion, plays an important role in diseases. | ||
* Mistargeting of the miRNAs, resulting in inhibition or activation of various [[oncogenes]], [[Tumor suppressor|tumor suppressors]], and other factors important in [[tumor]] [[Angiogenesis|angiogenesis.]] | * Mistargeting of the miRNAs, resulting in inhibition or activation of various [[oncogenes]], [[Tumor suppressor|tumor suppressors]], and other factors important in [[tumor]] [[Angiogenesis|angiogenesis.]]<ref name="pmid22337054">{{cite journal| author=Lujambio A, Lowe SW| title=The microcosmos of cancer. | journal=Nature | year= 2012 | volume= 482 | issue= 7385 | pages= 347-55 | pmid=22337054 | doi=10.1038/nature10888 | pmc=3509753 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22337054 }}</ref> | ||
* The investigation identified 14 upregulated miRNAs and 9 downregulated miRNAs unique to ACC. | * The investigation identified 14 upregulated miRNAs and 9 downregulated miRNAs unique to ACC.<ref name="pmid19996210">{{cite journal| author=Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR et al.| title=miR-195 and miR-483-5p Identified as Predictors of Poor Prognosis in Adrenocortical Cancer. | journal=Clin Cancer Res | year= 2009 | volume= 15 | issue= 24 | pages= 7684-7692 | pmid=19996210 | doi=10.1158/1078-0432.CCR-09-1587 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19996210 }}</ref> | ||
* Upregulated miRNAs in ACCs included miR-184, miR-210, and miR-503. | * Upregulated miRNAs in ACCs included miR-184, miR-210, and miR-503. | ||
* Downregulated miRNAs included miR-214, miR-375, and miR-511. | * Downregulated miRNAs included miR-214, miR-375, and miR-511.<ref name="pmid19546168">{{cite journal| author=Tömböl Z, Szabó PM, Molnár V, Wiener Z, Tölgyesi G, Horányi J et al.| title=Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. | journal=Endocr Relat Cancer | year= 2009 | volume= 16 | issue= 3 | pages= 895-906 | pmid=19546168 | doi=10.1677/ERC-09-0096 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19546168 }}</ref> | ||
* Levels of miR-184, miR-503, and miR-511 are able to distinguish benign from [[malignant]] [[Adrenal tumor|adrenal tumors]]. | * Levels of miR-184, miR-503, and miR-511 are able to distinguish benign from [[malignant]] [[Adrenal tumor|adrenal tumors]].<ref name="pmid19546168" /> | ||
* MiR-483 was found to be significantly upregulated in pediatric ACCs. | * MiR-483 was found to be significantly upregulated in pediatric ACCs. | ||
* MiR-99a and miR-100 are bioinformatically predicted to target the 3- untranslated regions of ''IGF1R'', ''RPTOR'', and ''FRAP1'' and were experimentally confirmed to target several components of the [[IGF-1]] signaling pathway. | * MiR-99a and miR-100 are bioinformatically predicted to target the 3- untranslated regions of ''IGF1R'', ''RPTOR'', and ''FRAP1'' and were experimentally confirmed to target several components of the [[IGF-1]] signaling pathway.<ref name="pmid20484036">{{cite journal| author=Doghman M, El Wakil A, Cardinaud B, Thomas E, Wang J, Zhao W et al.| title=Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. | journal=Cancer Res | year= 2010 | volume= 70 | issue= 11 | pages= 4666-75 | pmid=20484036 | doi=10.1158/0008-5472.CAN-09-3970 | pmc=2880211 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20484036 }}</ref> | ||
==== 4. '''''[[Gene mutation|Gene mutations]]''''' ==== | ==== 4. '''''[[Gene mutation|Gene mutations]]''''' ==== | ||
* Targeted genetic analyses have identified somatic genetic changes in ''TP53'', ''MEN1'', ''IGF2'', ''IGF2R'', and ''p16''. | * Targeted genetic analyses have identified somatic genetic changes in ''TP53'', ''MEN1'', ''IGF2'', ''IGF2R'', and ''p16''.<ref name="pmid11454518">{{cite journal| author=Barzon L, Chilosi M, Fallo F, Martignoni G, Montagna L, Palù G et al.| title=Molecular analysis of CDKN1C and TP53 in sporadic adrenal tumors. | journal=Eur J Endocrinol | year= 2001 | volume= 145 | issue= 2 | pages= 207-12 | pmid=11454518 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11454518 }}</ref> | ||
* ''TP53'' located on 17p13 is the most commonly mutated gene in ACC, present in at least one-third ofACCs | * ''TP53'' located on 17p13 is the most commonly mutated gene in ACC, present in at least one-third ofACCs.<ref name="pmid22504887">{{cite journal| author=Jain M, Rechache N, Kebebew E| title=Molecular markers of adrenocortical tumors. | journal=J Surg Oncol | year= 2012 | volume= 106 | issue= 5 | pages= 549-56 | pmid=22504887 | doi=10.1002/jso.23119 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22504887 }}</ref> | ||
* LOH in the gene encoding p16ink/p14arf, ''CDKN2A'' is observed in a subset of ACCs. The tumor suppressor function of this gene has been established in multiple cancers | * LOH in the gene encoding p16ink/p14arf, ''CDKN2A'' is observed in a subset of ACCs. The tumor suppressor function of this gene has been established in multiple cancers. LOH of 11q13 has been identified in 83% of samples.<ref name="pmid10022445">{{cite journal| author=Kjellman M, Roshani L, Teh BT, Kallioniemi OP, Höög A, Gray S et al.| title=Genotyping of adrenocortical tumors: very frequent deletions of the MEN1 locus in 11q13 and of a 1-centimorgan region in 2p16. | journal=J Clin Endocrinol Metab | year= 1999 | volume= 84 | issue= 2 | pages= 730-5 | pmid=10022445 | doi=10.1210/jcem.84.2.5506 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10022445 }}</ref> | ||
* ''MEN1'' (located on 11q13) somatic mutations are unusual in sporadic ACC. | * ''MEN1'' (located on 11q13) somatic mutations are unusual in sporadic ACC.<ref name="pmid17854394">{{cite journal| author=Tadjine M, Lampron A, Ouadi L, Bourdeau I| title=Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. | journal=Clin Endocrinol (Oxf) | year= 2008 | volume= 68 | issue= 2 | pages= 264-70 | pmid=17854394 | doi=10.1111/j.1365-2265.2007.03033.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17854394 }}</ref> | ||
* The canonical Wnt pathway, the catenin gene, and ''CTNNB1'' have been identified as activating point mutations in over 25% of both ACAs and ACCs in children and adults. | * The canonical Wnt pathway, the catenin gene, and ''CTNNB1'' have been identified as activating point mutations in over 25% of both ACAs and ACCs in children and adults.<ref name="pmid18647815">{{cite journal| author=Gaujoux S, Tissier F, Groussin L, Libé R, Ragazzon B, Launay P et al.| title=Wnt/beta-catenin and 3',5'-cyclic adenosine 5'-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors. | journal=J Clin Endocrinol Metab | year= 2008 | volume= 93 | issue= 10 | pages= 4135-40 | pmid=18647815 | doi=10.1210/jc.2008-0631 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18647815 }}</ref> | ||
==== 5. '''''[[Chromosomal aberration|Chromosomal aberrations]]''''' ==== | ==== 5. '''''[[Chromosomal aberration|Chromosomal aberrations]]''''' ==== | ||
* [[Comparative genomic hybridization]]([[Comparative genomic hybridization|CGH]]) can identify structural [[chromosomal]] abnormalities within ACCs. | * [[Comparative genomic hybridization]]([[Comparative genomic hybridization|CGH]]) can identify structural [[chromosomal]] abnormalities within ACCs.<ref name="pmid23093492">{{cite journal| author=Barreau O, Assié G, Wilmot-Roussel H, Ragazzon B, Baudry C, Perlemoine K et al.| title=Identification of a CpG island methylator phenotype in adrenocortical carcinomas. | journal=J Clin Endocrinol Metab | year= 2013 | volume= 98 | issue= 1 | pages= E174-84 | pmid=23093492 | doi=10.1210/jc.2012-2993 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23093492 }}</ref> | ||
* ACCs showed complex chromosomal alterations. ACCs contained multiple chromosomal gains or losses with a mean of 10 events. | * ACCs showed complex chromosomal alterations. ACCs contained multiple chromosomal gains or losses with a mean of 10 events. | ||
* The newest study confirmed increased alterations in ACC (44%) compared with ACAs (10%). | * The newest study confirmed increased alterations in ACC (44%) compared with ACAs (10%). | ||
* In ACCs, the frequently observed [[chromosomal]] gains at 5, 7, 12, 16, 19, and 20 and losses at 13 and 22 were confirmed. | * In ACCs, the frequently observed [[chromosomal]] gains at 5, 7, 12, 16, 19, and 20 and losses at 13 and 22 were confirmed. | ||
* The group identified genes within these regions with potential tumorigenic potential including fibroblast growth factor 4 (''FGF4''), cyclin-dependent kinase 4 (''CDK4''), and cyclin E1 ([[CCNE1|''CCNE1'')]]. The study confirmed the diagnostic utility of 6 [[loci]] (5q, 7p, 11p, 13q, 16q, and 22q) in the differentiation of ACA and ACC | * The group identified genes within these regions with potential tumorigenic potential including fibroblast growth factor 4 (''FGF4''), cyclin-dependent kinase 4 (''CDK4''), and cyclin E1 ([[CCNE1|''CCNE1'')]]. The study confirmed the diagnostic utility of 6 [[loci]] (5q, 7p, 11p, 13q, 16q, and 22q) in the differentiation of ACA and ACC. | ||
* [[Genomic]] aberration at [[chromosomes]] 5, 12, and 17 are predicted to illustrate [[genes]] that initiate or maintain [[Neoplasm|neoplastic]] transformation. [[Chromosome]] 17, specifically at 17p13, contains the well-known [[tumor suppressor gene]] ''[[TP53 (gene)|TP53]]''. | * [[Genomic]] aberration at [[chromosomes]] 5, 12, and 17 are predicted to illustrate [[genes]] that initiate or maintain [[Neoplasm|neoplastic]] transformation. [[Chromosome]] 17, specifically at 17p13, contains the well-known [[tumor suppressor gene]] ''[[TP53 (gene)|TP53]]''. | ||
=== 6. '''''[[Epigenetics|Epigenetic]] changes''''' === | === 6. '''''[[Epigenetics|Epigenetic]] changes''''' === | ||
* [[DNA methylation]] involves the addition of a [[methyl group]] to the [[cytosine]] [[pyrimidine]] ring or [[adenine]] [[purine]] ring. | * [[DNA methylation]] involves the addition of a [[methyl group]] to the [[cytosine]] [[pyrimidine]] ring or [[adenine]] [[purine]] ring.<ref name="pmid25111790">{{cite journal| author=Hofland J, Steenbergen J, Voorsluijs JM, Verbiest MM, de Krijger RR, Hofland LJ et al.| title=Inhibin alpha-subunit (INHA) expression in adrenocortical cancer is linked to genetic and epigenetic INHA promoter variation. | journal=PLoS One | year= 2014 | volume= 9 | issue= 8 | pages= e104944 | pmid=25111790 | doi=10.1371/journal.pone.0104944 | pmc=4128726 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25111790 }}</ref> | ||
* Dysregulation in this process is observed in [[Tumor cell|tumor cells.]] | * Dysregulation in this process is observed in [[Tumor cell|tumor cells.]] | ||
* A recent study revealed [[Methylation|hypermethylation]] of promoters in ACC with correlation to poor survival and identified ''[[H19 (gene)|H19]]'', ''[[PLAGL1]]'', ''[[G0 phase|G0S2]]'', and ''[[NDRG2]]'' as silenced genes also provided evidence about the role of [[methylation]] in ACC [[tumorigenesis]], particularly in the 11p15 [[locus]] containing ''[[IGF2]]'' and ''[[H19 (gene)|H19]]''. | * A recent study revealed [[Methylation|hypermethylation]] of promoters in ACC with correlation to poor survival and identified ''[[H19 (gene)|H19]]'', ''[[PLAGL1]]'', ''[[G0 phase|G0S2]]'', and ''[[NDRG2]]'' as silenced genes also provided evidence about the role of [[methylation]] in ACC [[tumorigenesis]], particularly in the 11p15 [[locus]] containing ''[[IGF2]]'' and ''[[H19 (gene)|H19]]''. | ||
| Line 85: | Line 80: | ||
==== '''''1. [[IGF]] pathway'''''<nowiki/> ==== | ==== '''''1. [[IGF]] pathway'''''<nowiki/> ==== | ||
* In the adult adrenal cortex, both IGF-1 and IGF-2 stimulate basal and ACTH-induced steroidogenesis | * In the adult adrenal cortex, both IGF-1 and IGF-2 stimulate basal and ACTH-induced steroidogenesis.<ref name="pmid3031644">{{cite journal| author=Voutilainen R, Miller WL| title=Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain-cleavage enzyme, P450scc [corrected], in human steroidogenic tissues. | journal=Proc Natl Acad Sci U S A | year= 1987 | volume= 84 | issue= 6 | pages= 1590-4 | pmid=3031644 | doi= | pmc=304481 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3031644 }}</ref> | ||
* Overall, the main role of IGF-2 lies in fetal development and growth, whereas IGF-1 acts mainly postnatally. | * Overall, the main role of IGF-2 lies in fetal development and growth, whereas IGF-1 acts mainly postnatally.<ref name="pmid1446644">{{cite journal| author=Han VK, Lu F, Bassett N, Yang KP, Delhanty PJ, Challis JR| title=Insulin-like growth factor-II (IGF-II) messenger ribonucleic acid is expressed in steroidogenic cells of the developing ovine adrenal gland: evidence of an autocrine/paracrine role for IGF-II. | journal=Endocrinology | year= 1992 | volume= 131 | issue= 6 | pages= 3100-9 | pmid=1446644 | doi=10.1210/endo.131.6.1446644 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1446644 }}</ref> | ||
* Prominent overexpression of ''IGF2'' and alterations of the ''IGF2''/''H19'' locus have been identified in sporadic ACC | * Prominent overexpression of ''IGF2'' and alterations of the ''IGF2''/''H19'' locus have been identified in sporadic ACC.<ref name="pmid12547710">{{cite journal| author=Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL et al.| title=Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. | journal=Am J Pathol | year= 2003 | volume= 162 | issue= 2 | pages= 521-31 | pmid=12547710 | doi=10.1016/S0002-9440(10)63846-1 | pmc=1851158 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12547710 }}</ref> | ||
* The ''IGF2'' gene is located on 11p15, which also includes a noncoding ''H19'' gene and a cyclin-dependent kinase inhibitor, ''CDKN1C'' (''p57KIP2'') (216, 217), and 80% to 90% of all ACCs show very high ''IGF2'' expression (174, 218– 220). Pediatric ACCs reveal a 20-fold overexpression of ''IGF2''. | * The ''IGF2'' gene is located on 11p15, which also includes a noncoding ''H19'' gene and a cyclin-dependent kinase inhibitor, ''CDKN1C'' (''p57KIP2'') (216, 217), and 80% to 90% of all ACCs show very high ''IGF2'' expression (174, 218– 220). Pediatric ACCs reveal a 20-fold overexpression of ''IGF2''.<ref name="pmid11436121">{{cite journal| author=Gaston V, Le Bouc Y, Soupre V, Burglen L, Donadieu J, Oro H et al.| title=Analysis of the methylation status of the KCNQ1OT and H19 genes in leukocyte DNA for the diagnosis and prognosis of Beckwith-Wiedemann syndrome. | journal=Eur J Hum Genet | year= 2001 | volume= 9 | issue= 6 | pages= 409-18 | pmid=11436121 | doi=10.1038/sj.ejhg.5200649 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11436121 }}</ref> | ||
* Patients with high ''IGF2'' expression levels and 11p15 LOH are associated with a 5-fold increased risk for recurrence and a shorter disease-free survival | * Patients with high ''IGF2'' expression levels and 11p15 LOH are associated with a 5-fold increased risk for recurrence and a shorter disease-free survival.<ref name="pmid18854392">{{cite journal| author=Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG et al.| title=Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. | journal=J Clin Endocrinol Metab | year= 2009 | volume= 94 | issue= 1 | pages= 204-12 | pmid=18854392 | doi=10.1210/jc.2008-1456 | pmc=2630877 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18854392 }}</ref> | ||
* loss of maternally expressed ''CDKN1C'' and ''H19'', may contribute to adrenal tumorigenesis | * loss of maternally expressed ''CDKN1C'' and ''H19'', may contribute to adrenal tumorigenesis.<ref name="pmid11559548">{{cite journal| author=Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E et al.| title=Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. | journal=Cancer Res | year= 2001 | volume= 61 | issue= 18 | pages= 6762-7 | pmid=11559548 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11559548 }}</ref> | ||
* | * The combined treatment of NCI-H295 cells with IGF-1R antagonists and mitotane resulted in a synergistic antiproliferative effect in vitro and in vivo in tumor xenografts.<ref name="pmid18611974">{{cite journal| author=Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH et al.| title=Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. | journal=J Clin Endocrinol Metab | year= 2008 | volume= 93 | issue= 9 | pages= 3524-31 | pmid=18611974 | doi=10.1210/jc.2008-0065 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18611974 }}</ref> | ||
'''''2. WNT signaling pathway''''' | '''''2. WNT signaling pathway''''' | ||
* The WNT/ catenin signaling pathway is a major developmental pathway in multiple organ systems, including the adrenal gland. (228) | * The WNT/ catenin signaling pathway is a major developmental pathway in multiple organ systems, including the adrenal gland. (228) | ||
Revision as of 21:08, 21 September 2017
|
Adrenocortical carcinoma Microchapters |
|
Differentiating Adrenocortical carcinoma from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Study |
|
Adrenocortical carcinoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Adrenocortical carcinoma pathophysiology |
|
Risk calculators and risk factors for Adrenocortical carcinoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2]Shivali Marketkar, M.B.B.S. [3]Ahmad Al Maradni, M.D. [4]
Overview
On gross pathology, a large tan-yellow surface with areas of hemorrhage and necrosis is a characteristic finding of adrenocortical carcinoma. On microscopic histopathological analysis, sheets of atypical cells with some resemblance to the cells of the normal adrenal cortex are a characteristic finding of adrenocortical carcinoma.
Pathophysiology
- ACCs are typically large tumors upon clinical presentation, often measuring more than 6 cm in diameter.[1]
- They are bilateral in 2% to 10% of cases.
- Metastases to the liver, lungs, or lymph nodes can be seen, and invasion of adjacent organs or venous extension into the renal vein and/or inferior vena cava may be present.[2]
- Inferior vena cava invasion has been reported in 9% to 19% of cases at presentation.[3]
- Due to the presence of internal hemorrhage, necrosis, and calcifications, these tumors tend to vary in appearance with frequent heterogeneous enhancement.
Spread can take several forms:[4]
- Direct invasion of the tumor capsule, invasion through the tumor capsule into extra-adrenal soft tissue
- Direct invasion of lymphatic vessels in and around the capsule and near blood vessels.
- Metastatic deposits are largely similar to the primary tumor
ACCs can be graded into low- and high-grade carcinoma groups based on their mitotic rates ( >20 mitoses per 50 high-power fields vs. <20 mitoses per 50 high-power fields)
Mitotic rate was most closely associated with patient outcome.
ACCs in children behave in a more indolent fashion compared with the adult, that is why there are so many pediatric ACCs but few pediatric deaths.[5]
Genetics
The genetic dissection of ACC has revealed genomic aberrations that contribute to neoplastic transformation of adrenocortical cells:
1. Clonality
- ACCs initiate from monoclonal cell populations, suggesting that mutation events lead to clonal expansion and ultimate progression to cancer.[6]
- Flow cytometry revealed aneuploidy in ACC. aneuploidy was observed in 75% of ACC.[7]
- Assessment of aneuploidy with histopathological criteria in 7 of 9 adrenal tumors revealed a high correlation with Weiss score >3 (indicative of malignancy).[8]
- No significant difference in overall survival was observed in patients with ACC exhibiting aneuploidy vs patients with ACC exhibiting diploid neoplasms.[9]
- An initial study identified elevated expression of genes involved in cell proliferation in ACC, such as IGF2, compared with increased expression of steroidogenic genes in ACA.[10]
- Giordano et al identified unique transcriptionally activated (12q and 5q) and repressed (11q, 1p, and 17p) chromosomal regions in 33 ACCs vs 22 ACAs in a microarray study.[11]
- Giordano et al (192) determined that ACC with high histological grade exhibited overexpression of cell cycle and functional aneuploidy genes and leading to the decreased survival of patients.
3. MicroRNAs
- MicroRNAs are RNAs that are important in the regulation of gene expression.
- Numerous miRNAs have been identified in the regulation of various cellular processes such as proliferation, apoptosis, and differentiation.[13]
- Dysregulation of miRNAs, such as overexpression or deletion, plays an important role in diseases.
- Mistargeting of the miRNAs, resulting in inhibition or activation of various oncogenes, tumor suppressors, and other factors important in tumor angiogenesis.[14]
- The investigation identified 14 upregulated miRNAs and 9 downregulated miRNAs unique to ACC.[15]
- Upregulated miRNAs in ACCs included miR-184, miR-210, and miR-503.
- Downregulated miRNAs included miR-214, miR-375, and miR-511.[16]
- Levels of miR-184, miR-503, and miR-511 are able to distinguish benign from malignant adrenal tumors.[16]
- MiR-483 was found to be significantly upregulated in pediatric ACCs.
- MiR-99a and miR-100 are bioinformatically predicted to target the 3- untranslated regions of IGF1R, RPTOR, and FRAP1 and were experimentally confirmed to target several components of the IGF-1 signaling pathway.[17]
4. Gene mutations
- Targeted genetic analyses have identified somatic genetic changes in TP53, MEN1, IGF2, IGF2R, and p16.[18]
- TP53 located on 17p13 is the most commonly mutated gene in ACC, present in at least one-third ofACCs.[19]
- LOH in the gene encoding p16ink/p14arf, CDKN2A is observed in a subset of ACCs. The tumor suppressor function of this gene has been established in multiple cancers. LOH of 11q13 has been identified in 83% of samples.[20]
- MEN1 (located on 11q13) somatic mutations are unusual in sporadic ACC.[21]
- The canonical Wnt pathway, the catenin gene, and CTNNB1 have been identified as activating point mutations in over 25% of both ACAs and ACCs in children and adults.[22]
5. Chromosomal aberrations
- Comparative genomic hybridization(CGH) can identify structural chromosomal abnormalities within ACCs.[23]
- ACCs showed complex chromosomal alterations. ACCs contained multiple chromosomal gains or losses with a mean of 10 events.
- The newest study confirmed increased alterations in ACC (44%) compared with ACAs (10%).
- In ACCs, the frequently observed chromosomal gains at 5, 7, 12, 16, 19, and 20 and losses at 13 and 22 were confirmed.
- The group identified genes within these regions with potential tumorigenic potential including fibroblast growth factor 4 (FGF4), cyclin-dependent kinase 4 (CDK4), and cyclin E1 (CCNE1). The study confirmed the diagnostic utility of 6 loci (5q, 7p, 11p, 13q, 16q, and 22q) in the differentiation of ACA and ACC.
- Genomic aberration at chromosomes 5, 12, and 17 are predicted to illustrate genes that initiate or maintain neoplastic transformation. Chromosome 17, specifically at 17p13, contains the well-known tumor suppressor gene TP53.
6. Epigenetic changes
- DNA methylation involves the addition of a methyl group to the cytosine pyrimidine ring or adenine purine ring.[24]
- Dysregulation in this process is observed in tumor cells.
- A recent study revealed hypermethylation of promoters in ACC with correlation to poor survival and identified H19, PLAGL1, G0S2, and NDRG2 as silenced genes also provided evidence about the role of methylation in ACC tumorigenesis, particularly in the 11p15 locus containing IGF2 and H19.
Cellular signaling pathway
1. IGF pathway
- In the adult adrenal cortex, both IGF-1 and IGF-2 stimulate basal and ACTH-induced steroidogenesis.[25]
- Overall, the main role of IGF-2 lies in fetal development and growth, whereas IGF-1 acts mainly postnatally.[26]
- Prominent overexpression of IGF2 and alterations of the IGF2/H19 locus have been identified in sporadic ACC.[27]
- The IGF2 gene is located on 11p15, which also includes a noncoding H19 gene and a cyclin-dependent kinase inhibitor, CDKN1C (p57KIP2) (216, 217), and 80% to 90% of all ACCs show very high IGF2 expression (174, 218– 220). Pediatric ACCs reveal a 20-fold overexpression of IGF2.[28]
- Patients with high IGF2 expression levels and 11p15 LOH are associated with a 5-fold increased risk for recurrence and a shorter disease-free survival.[29]
- loss of maternally expressed CDKN1C and H19, may contribute to adrenal tumorigenesis.[30]
- The combined treatment of NCI-H295 cells with IGF-1R antagonists and mitotane resulted in a synergistic antiproliferative effect in vitro and in vivo in tumor xenografts.[31]
2. WNT signaling pathway
- The WNT/ catenin signaling pathway is a major developmental pathway in multiple organ systems, including the adrenal gland. (228)
- The pathway is differentiated into 3 diverging signaling cascades dependent on signal conduction through:
- Catenin (canonical pathway)
- Ras homolog gene family small GTPase (planar cell polarity pathway)
- Phospholipase C (Wnt/calcium pathway)
- Initial alterations of the WNT/ catenin system/pathway were identified in FAP (229, 230).
- Recent examinations of adrenocortical tumors suggest that the WNT/_-catenin signaling pathway plays an important role in sporadic adrenocortical tumorigenesis.
- Immunohistochemical analysis of 39 adrenal tumors revealed accumulation of catenin in ACCs. (149)
- Mutational analysis of the catenin gene CTNNB1 identified activating point mutations in ACCs (149, 206–208).
- Inactivating mutations of AXIN2 (a component of the catenin destruction complex) have also been described in some adrenocortical tumors (231).
- Both nuclear catenin accumulation and activating CTNNB1 mutations are present in ACCs suggests that WNT activation is a part of ACA tumorigenesis.
3. Vascular endothelial growth factor
- The vascular endothelial growth factor (VEGF) is a chief regulator of cancer angiogenesis. Its effects are mediated through its receptors (VEGFRs) (235).
- Elevated VEGF levels were identified in blood samples from ACC patients (237, 238).
- Overexpression of VEGFR type 2 in ACC samples was observed by immunohistochemistry (239).
- The increased expression of VEGF correlates with the expression of IGF2 (192).
- The pharmacological inhibition of VEGFRs is considered an attractive option for cancer treatment (236).
Hormones biosynthesis in adrenal cortex
- Cortisol is synthesized from cholesterol. Synthesis takes place in the zona fasciculata of the adrenal cortex.
- Aldosterone is produced in the zona glomerulosa
- Sex hormones are synthesized in in the zona reticularis
- The secretion of cortisol is controlled by hypothalamic-pituitary axis by the following mechanism:[1][2]
Paraventricular nuclei in the hypothalamus release corticotropin releasing hormone (CRH).
CRH is transferred to anterior pituitary via the portal veins.
CRH stimulates the activity of corticotrophs; cells that produce proopiomelanocortin (POMC) in the anterior pituitary.
Corticotrophs produce adrenocorticotropic hormone (ACTH) by the post-translational modification of POMC.
ACTH is drained into systemic circulation via the pituitary capillaries and stimulates the adrenal cortex (zona fasciculata) to produce cortisol.
Cortisol acts on hypothalamus and pituitary through a feedback mechanism to regulate the secretion of CRH and ACTH.
Gross Pathology
On gross pathology, adrenocortical carcinomas are often large (>5 cm in largest diameter), with a tan-yellow cut surface and areas of hemorrhage and necrosis.
Their cut surface ranges from brown to orange to yellow depending on the lipid content of their cells. Necrosis is almost always present.
Typical ACC with a hypercellular population of cells with the earliest form of tumor necrosis.
A typical ACC with a solid growth pattern and abundant eosinophilic cytoplasm with focal clear areas, consistent with lipid.
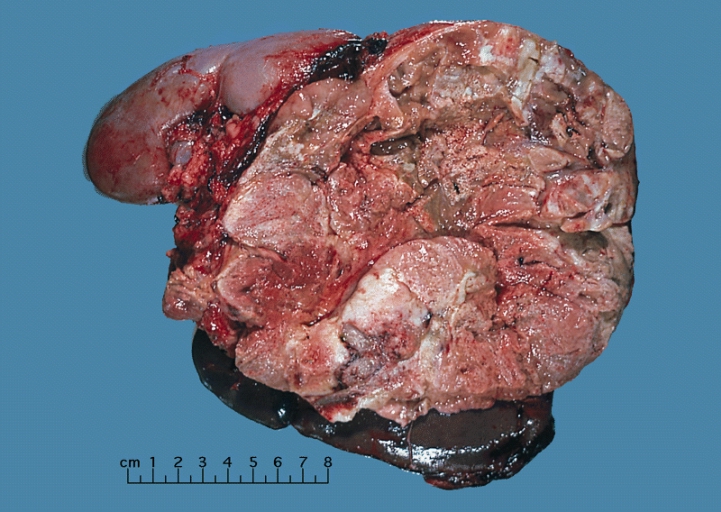
Shown above is a large adrenal cortical carcinoma resected from a 27-year-old woman. The tumor measured 17 cm in diameter and invaded kidney and spleen which necessitated en bloc removal of these organs with tumor. Patient had evidence of virilization.
Microscopic Pathology
On microscopic examination, the tumor usually displays sheets of atypical cells with some resemblance to the cells of the normal adrenal cortex. The presence of invasion and mitotic activity helps differentiate small cancers from adrenocortical adenomas.[32]
The Weiss criteria of adrenocortical malignancy is the most reliable histopathological scoring system differentiating ACC from adrenocortical adenoma.
ACC can be diagnosed by the presence of at least 3 of the 9 Weiss criteria:
- Nuclear grade III or IV based on Fuhrman criteria
- More than 5 mitotic figures/50 HPF (40x objective), counting 10 random fields in area of greatest number of mitotic figures on 5 slides with the greatest number of mitosis
- Presence of atypical mitotic figures (abnormal distribution of chromosomes or excessive number of mitotic spindles)
- Clear or vacuolated cells comprising 25% or less of tumor
- Diffuse architecture (more than 1/3 of tumor forms patternless sheets of cells; trabecular, cord, columnar, alveolar or nesting pattern is not considered to be diffuse)
- Microscopic necrosis
- Venous invasion (veins must have smooth muscle in wall; tumor cell clusters or sheets forming polypoid projections into vessel lumen or polypoid tumor thrombi covered by endothelial layer)
- Sinusoidal invasion (sinusoid is endothelial lined vessel in adrenal gland with little supportive tissue; consider only sinusoids within tumor)
- Capsular invasion (nests or cords of tumor extending into or through capsule with a stromal reaction); either incomplete or complete
Modified Weiss criteria (score of 3 or more suggests malignancy):
- Mitotic rate >5 per 50 high-power fields
- Cytoplasm (clear cells comprising 25% or less of the tumor)
- Abnormal mitoses
- Necrosis
- Capsular invasion
- Calculate: 2x mitotic rate criterion + 2x clear cytoplasm criterion + abnormal mitoses + necrosis + capsular invasion
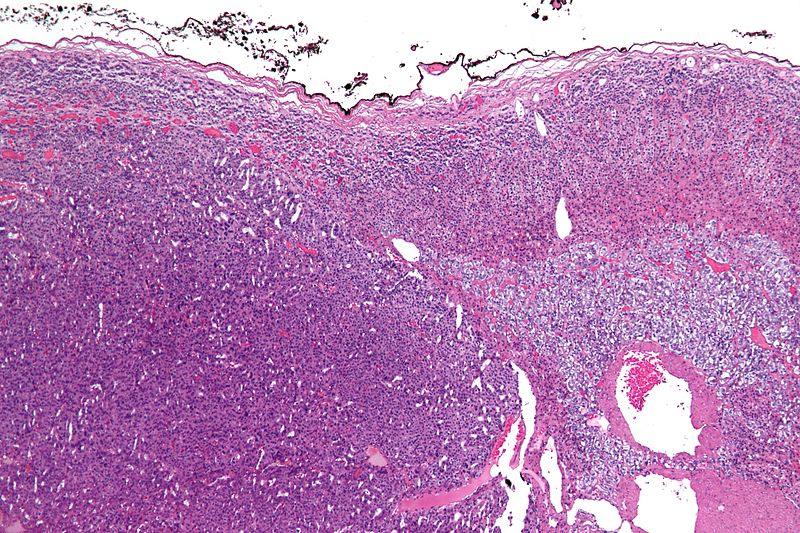
Micrograph of an adrenocortical carcinoma (left of image - dark blue) and the adrenal cortex it arose from (right-top of image - pink/light blue). Benign adrenal medulla is present (right-middle of image - gray/blue). H&E stain.
Video
Shown below is a video explaining the histology of adrenocortical carcinoma
{{#ev:youtube|7jMFENhPaOM}}
References
- ↑ Johnson PT, Horton KM, Fishman EK (2009). "Adrenal mass imaging with multidetector CT: pathologic conditions, pearls, and pitfalls". Radiographics. 29 (5): 1333–51. doi:10.1148/rg.295095027. PMID 19755599.
- ↑ Dunnick NR, Heaston D, Halvorsen R, Moore AV, Korobkin M (1982). "CT appearance of adrenal cortical carcinoma". J Comput Assist Tomogr. 6 (5): 978–82. PMID 7142516.
- ↑ Bharwani N, Rockall AG, Sahdev A, Gueorguiev M, Drake W, Grossman AB; et al. (2011). "Adrenocortical carcinoma: the range of appearances on CT and MRI". AJR Am J Roentgenol. 196 (6): W706–14. doi:10.2214/AJR.10.5540. PMID 21606258.
- ↑ Dehner LP, Hill DA (2009). "Adrenal cortical neoplasms in children: why so many carcinomas and yet so many survivors?". Pediatr Dev Pathol. 12 (4): 284–91. doi:10.2350/08-06-0489.1. PMID 19326954.
- ↑ Cagle PT, Hough AJ, Pysher TJ, Page DL, Johnson EH, Kirkland RT; et al. (1986). "Comparison of adrenal cortical tumors in children and adults". Cancer. 57 (11): 2235–7. PMID 3697922.
- ↑ Beuschlein F, Reincke M, Karl M, Travis WD, Jaursch-Hancke C, Abdelhamid S; et al. (1994). "Clonal composition of human adrenocortical neoplasms". Cancer Res. 54 (18): 4927–32. PMID 7915195.
- ↑ Gicquel C, Leblond-Francillard M, Bertagna X, Louvel A, Chapuis Y, Luton JP; et al. (1994). "Clonal analysis of human adrenocortical carcinomas and secreting adenomas". Clin Endocrinol (Oxf). 40 (4): 465–77. PMID 7910530.
- ↑ Amberson JB, Vaughan ED, Gray GF, Naus GJ (1987). "Flow cytometric determination of nuclear DNA content in benign adrenal pheochromocytomas". Urology. 30 (2): 102–4. PMID 3617290.
- ↑ Cibas ES, Medeiros LJ, Weinberg DS, Gelb AB, Weiss LM (1990). "Cellular DNA profiles of benign and malignant adrenocortical tumors". Am J Surg Pathol. 14 (10): 948–55. PMID 2403197.
- ↑ de Fraipont F, El Atifi M, Cherradi N, Le Moigne G, Defaye G, Houlgatte R; et al. (2005). "Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic Acid microarrays identifies several candidate genes as markers of malignancy". J Clin Endocrinol Metab. 90 (3): 1819–29. doi:10.1210/jc.2004-1075. PMID 15613424.
- ↑ Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J; et al. (2009). "Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling". Clin Cancer Res. 15 (2): 668–76. doi:10.1158/1078-0432.CCR-08-1067. PMC 2629378. PMID 19147773.
- ↑ de Reyniès A, Assié G, Rickman DS, Tissier F, Groussin L, René-Corail F; et al. (2009). "Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival". J Clin Oncol. 27 (7): 1108–15. doi:10.1200/JCO.2008.18.5678. PMID 19139432.
- ↑ Czech B, Hannon GJ (2011). "Small RNA sorting: matchmaking for Argonautes". Nat Rev Genet. 12 (1): 19–31. doi:10.1038/nrg2916. PMC 3703915. PMID 21116305.
- ↑ Lujambio A, Lowe SW (2012). "The microcosmos of cancer". Nature. 482 (7385): 347–55. doi:10.1038/nature10888. PMC 3509753. PMID 22337054.
- ↑ Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR; et al. (2009). "miR-195 and miR-483-5p Identified as Predictors of Poor Prognosis in Adrenocortical Cancer". Clin Cancer Res. 15 (24): 7684–7692. doi:10.1158/1078-0432.CCR-09-1587. PMID 19996210.
- ↑ 16.0 16.1 Tömböl Z, Szabó PM, Molnár V, Wiener Z, Tölgyesi G, Horányi J; et al. (2009). "Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis". Endocr Relat Cancer. 16 (3): 895–906. doi:10.1677/ERC-09-0096. PMID 19546168.
- ↑ Doghman M, El Wakil A, Cardinaud B, Thomas E, Wang J, Zhao W; et al. (2010). "Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors". Cancer Res. 70 (11): 4666–75. doi:10.1158/0008-5472.CAN-09-3970. PMC 2880211. PMID 20484036.
- ↑ Barzon L, Chilosi M, Fallo F, Martignoni G, Montagna L, Palù G; et al. (2001). "Molecular analysis of CDKN1C and TP53 in sporadic adrenal tumors". Eur J Endocrinol. 145 (2): 207–12. PMID 11454518.
- ↑ Jain M, Rechache N, Kebebew E (2012). "Molecular markers of adrenocortical tumors". J Surg Oncol. 106 (5): 549–56. doi:10.1002/jso.23119. PMID 22504887.
- ↑ Kjellman M, Roshani L, Teh BT, Kallioniemi OP, Höög A, Gray S; et al. (1999). "Genotyping of adrenocortical tumors: very frequent deletions of the MEN1 locus in 11q13 and of a 1-centimorgan region in 2p16". J Clin Endocrinol Metab. 84 (2): 730–5. doi:10.1210/jcem.84.2.5506. PMID 10022445.
- ↑ Tadjine M, Lampron A, Ouadi L, Bourdeau I (2008). "Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas". Clin Endocrinol (Oxf). 68 (2): 264–70. doi:10.1111/j.1365-2265.2007.03033.x. PMID 17854394.
- ↑ Gaujoux S, Tissier F, Groussin L, Libé R, Ragazzon B, Launay P; et al. (2008). "Wnt/beta-catenin and 3',5'-cyclic adenosine 5'-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors". J Clin Endocrinol Metab. 93 (10): 4135–40. doi:10.1210/jc.2008-0631. PMID 18647815.
- ↑ Barreau O, Assié G, Wilmot-Roussel H, Ragazzon B, Baudry C, Perlemoine K; et al. (2013). "Identification of a CpG island methylator phenotype in adrenocortical carcinomas". J Clin Endocrinol Metab. 98 (1): E174–84. doi:10.1210/jc.2012-2993. PMID 23093492.
- ↑ Hofland J, Steenbergen J, Voorsluijs JM, Verbiest MM, de Krijger RR, Hofland LJ; et al. (2014). "Inhibin alpha-subunit (INHA) expression in adrenocortical cancer is linked to genetic and epigenetic INHA promoter variation". PLoS One. 9 (8): e104944. doi:10.1371/journal.pone.0104944. PMC 4128726. PMID 25111790.
- ↑ Voutilainen R, Miller WL (1987). "Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain-cleavage enzyme, P450scc [corrected], in human steroidogenic tissues". Proc Natl Acad Sci U S A. 84 (6): 1590–4. PMC 304481. PMID 3031644.
- ↑ Han VK, Lu F, Bassett N, Yang KP, Delhanty PJ, Challis JR (1992). "Insulin-like growth factor-II (IGF-II) messenger ribonucleic acid is expressed in steroidogenic cells of the developing ovine adrenal gland: evidence of an autocrine/paracrine role for IGF-II". Endocrinology. 131 (6): 3100–9. doi:10.1210/endo.131.6.1446644. PMID 1446644.
- ↑ Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL; et al. (2003). "Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis". Am J Pathol. 162 (2): 521–31. doi:10.1016/S0002-9440(10)63846-1. PMC 1851158. PMID 12547710.
- ↑ Gaston V, Le Bouc Y, Soupre V, Burglen L, Donadieu J, Oro H; et al. (2001). "Analysis of the methylation status of the KCNQ1OT and H19 genes in leukocyte DNA for the diagnosis and prognosis of Beckwith-Wiedemann syndrome". Eur J Hum Genet. 9 (6): 409–18. doi:10.1038/sj.ejhg.5200649. PMID 11436121.
- ↑ Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG; et al. (2009). "Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma". J Clin Endocrinol Metab. 94 (1): 204–12. doi:10.1210/jc.2008-1456. PMC 2630877. PMID 18854392.
- ↑ Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E; et al. (2001). "Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors". Cancer Res. 61 (18): 6762–7. PMID 11559548.
- ↑ Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH; et al. (2008). "Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors". J Clin Endocrinol Metab. 93 (9): 3524–31. doi:10.1210/jc.2008-0065. PMID 18611974.
- ↑ Richard Cote, Saul Suster, Lawrence Weiss, Noel Weidner (Editor). Modern Surgical Pathology (2 Volume Set). London: W B Saunders. ISBN 0-7216-7253-1.