Adrenocortical carcinoma pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
The genetic dissection of ACC has revealed genomic aberrations that contribute to neoplastic transformation of adrenocortical cells: | The genetic dissection of ACC has revealed genomic aberrations that contribute to neoplastic transformation of adrenocortical cells: | ||
'''''1. Clonality''''' | '''''1. [[Clone (cell biology)|Clonality]]''''' | ||
* ACCs initiate from monoclonal cell populations, suggesting that mutation events lead to clonal expansion and ultimate progression to cancer (177, 178). | * ACCs initiate from [[Monoclonal|monoclonal cell]] populations, suggesting that [[mutation]] events lead to [[Clonal selection|clonal expansion]] and ultimate progression to [[cancer]]. (177, 178). | ||
* Flow cytometry revealed aneuploidy in ACC.(180).aneuploidy was observed in 75% of ACC. | * [[Flow cytometry]] revealed [[aneuploidy]] in ACC.(180).[[aneuploidy]] was observed in 75% of ACC. | ||
* Assessment of aneuploidy with histopathological criteria in 7 of 9 adrenal tumors revealed a high correlation with Weiss score >3 (indicative of malignancy) (182). | * Assessment of [[aneuploidy]] with [[histopathological]] criteria in 7 of 9 [[Adrenal tumor|adrenal tumors]] revealed a high correlation with Weiss score >3 (indicative of [[malignancy]]) (182). | ||
* No significant difference in overall survival was observed in patients with ACC exhibiting aneuploidy vs patients with ACC exhibiting diploid neoplasms (126). | * No significant difference in overall survival was observed in patients with ACC exhibiting [[aneuploidy]] vs patients with ACC exhibiting [[Diploids|diploid]] [[Neoplasm|neoplasms]]. (126). | ||
'''''2. Gene expression arrays''''' | '''''2. [[Gene expression]] [[DNA microarray|arrays]]''''' | ||
* An initial study identified elevated [[Gene expression|expression of genes]] involved in cell proliferation in ACC, such as ''[[IGF2]]'', compared with increased [[Gene expression|expression]] of steroidogenic [[genes]] in ACA. (190). | |||
* Giordano et al identified unique [[Transcription (genetics)|transcriptionally]] activated (12q and 5q) and repressed (11q, 1p, and 17p) [[chromosomal]] regions in 33 ACCs vs 22 ACAs in a [[DNA microarray|microarray]] study.(192) | |||
* Giordano et al (192) determined that ACC with high [[histological]] [[Grading (tumors)|grade]] exhibited overexpression of [[cell cycle]] and functional [[aneuploidy]] [[genes]] and leading to the decreased survival of patients. | |||
* Expression levels of ''BUB1B,'' ''[[PINK1]], and [[DLG7]]'' ''are'' identified in ACC. (191). | |||
==== 3. '''''[[MicroRNAs]]''''' ==== | |||
* [[MicroRNAs]] are [[RNA|RNAs]] that are important in the regulation of [[gene expression]]. | |||
* Numerous [[MicroRNA|miRNAs]] have been identified in the regulation of various [[cellular]] processes such as [[proliferation]], [[Apoptosis|apoptosis,]] and [[differentiation]]. (194). | |||
* Dysregulation of miRNAs, such as overexpression or deletion, plays an important role in diseases. | |||
* Mistargeting of the miRNAs, resulting in inhibition or activation of various [[oncogenes]], [[Tumor suppressor|tumor suppressors]], and other factors important in [[tumor]] [[Angiogenesis|angiogenesis.]] (196). | |||
* The investigation identified 14 upregulated miRNAs and 9 downregulated miRNAs unique to ACC. (198). | |||
* Upregulated miRNAs in ACCs included miR-184, miR-210, and miR-503. | |||
* Downregulated miRNAs included miR-214, miR-375, and miR-511. (197). | |||
* Levels of miR-184, miR-503, and miR-511 are able to distinguish benign from [[malignant]] [[Adrenal tumor|adrenal tumors]]. (197). | |||
* MiR-483 was found to be significantly upregulated in pediatric ACCs. | |||
* MiR-99a and miR-100 are bioinformatically predicted to target the 3- untranslated regions of ''IGF1R'', ''RPTOR'', and ''FRAP1'' and were experimentally confirmed to target several components of the [[IGF-1]] signaling pathway. (199) | |||
==== 4. '''''[[Gene mutation|Gene mutations]]''''' ==== | |||
* Targeted genetic analyses have identified somatic genetic changes in ''TP53'', ''MEN1'', ''IGF2'', ''IGF2R'', and ''p16''. | |||
* ''TP53'' located on 17p13 is the most commonly mutated gene in ACC, present in at least one-third ofACCs (140, 142, 202) (203). | |||
* LOH in the gene encoding p16ink/p14arf, ''CDKN2A'' is observed in a subset of ACCs. The tumor suppressor function of this gene has been established in multiple cancers (204). LOH of 11q13 has been identified in 83% of samples. (185) | |||
* ''MEN1'' (located on 11q13) somatic mutations are unusual in sporadic ACC. | |||
* The canonical Wnt pathway, the catenin gene, and ''CTNNB1'' have been identified as activating point mutations in over 25% of both ACAs and ACCs in children and adults. (149, 206–208). | |||
==== 5. '''''[[Chromosomal aberration|Chromosomal aberrations]]''''' ==== | |||
* [[Comparative genomic hybridization]]([[Comparative genomic hybridization|CGH]]) can identify structural [[chromosomal]] abnormalities within ACCs. | |||
* ACCs showed complex chromosomal alterations. ACCs contained multiple chromosomal gains or losses with a mean of 10 events. | |||
* The newest study confirmed increased alterations in ACC (44%) compared with ACAs (10%). 188 | |||
* In ACCs, the frequently observed [[chromosomal]] gains at 5, 7, 12, 16, 19, and 20 and losses at 13 and 22 were confirmed. | |||
* The group identified genes within these regions with potential tumorigenic potential including fibroblast growth factor 4 (''FGF4''), cyclin-dependent kinase 4 (''CDK4''), and cyclin E1 ([[CCNE1|''CCNE1'')]]. The study confirmed the diagnostic utility of 6 [[loci]] (5q, 7p, 11p, 13q, 16q, and 22q) in the differentiation of ACA and ACC(188). | |||
* [[Genomic]] aberration at [[chromosomes]] 5, 12, and 17 are predicted to illustrate [[genes]] that initiate or maintain [[Neoplasm|neoplastic]] transformation. [[Chromosome]] 17, specifically at 17p13, contains the well-known [[tumor suppressor gene]] ''[[TP53 (gene)|TP53]]''. | |||
=== 6. '''''[[Epigenetics|Epigenetic]] changes''''' === | |||
* [[DNA methylation]] involves the addition of a [[methyl group]] to the [[cytosine]] [[pyrimidine]] ring or [[adenine]] [[purine]] ring. | |||
* Dysregulation in this process is observed in [[Tumor cell|tumor cells.]] | |||
* A recent study revealed [[Methylation|hypermethylation]] of promoters in ACCs with correlation to poor survival and identified ''[[H19 (gene)|H19]]'', ''[[PLAGL1]]'', ''[[G0 phase|G0S2]]'', and ''[[NDRG2]]'' as silenced genes also provided evidence about the role of [[methylation]] in ACC [[tumorigenesis]], particularly in the 11p15 [[locus]] containing ''[[IGF2]]'' and ''[[H19 (gene)|H19]]''. | |||
The [[IGF]] signaling pathway consists of [[ligand]]<nowiki/>s ([[IGF-1]] and [[IGF2|IGF-2]]), receptors (IGF-1 receptor [IGF-1R], IGF-2R, and insulin receptor), IGF binding proteins 1–6, and IGF binding protein proteases. | |||
The binding of the mitogenic polypeptides to their receptors activates the downstream AKT/PI3K and MAPK pathways to regulate cellular processes of metabolism, differentiation, proliferation, and apoptosis. | |||
The IGF pathway mediates ACTH-induced prenatal adrenal growth, fetal and adult steroidogenesis, and organ maintenance (209–212). | |||
In the developing fetal organ, ''IGF1'' expression is restricted to the capsule, whereas ''IGF2'' expression is enriched in the cortex (213). | |||
In the adult adrenal cortex, both IGF-1 and IGF-2 stimulate basal and ACTH-induced steroidogenesis (210, 214). Overall, the main role of IGF-2 lies in fetal development and growth, whereas IGF-1 acts mainly postnatally. Prominent overexpression of ''IGF2'' and alterations of the ''IGF2''/''H19'' locus have been identified in sporadic ACC(174, 190, 215). The ''IGF2'' gene is located on 11p15, which also includes a noncoding ''H19'' gene and a cyclindependent kinase inhibitor, ''CDKN1C'' (''p57KIP2'') (216, 217), and 80% to 90% of all ACCs show very high ''IGF2'' expression (_100-fold over normal and ACA) (174, 218– 220). Interestingly, relative expression of ''Igf2'' is much higher than in tissues from mice resembling human BWS, in which genetic changes result in an _2-fold upregulation. High ''IGF2'' expression levels in adrenal tumors, when analyzing malignant and benign tumors, are associated with a 5-fold increased risk for recurrence and a shorter disease-free survival (184, 191). Pediatric ACCs reveal an _20-fold overexpression of ''IGF2''. Various cell culture studies using ACC cell lines suggest a paracrine or autocrine effect of IGF-2 and mitogenic activity through IGF-1R (156, 221–223). ''PEPCK''-''IGF2'' transgenic mice that overexpress ''IGF2'' have adrenocortical hyperplasia and enhanced steroidogenesis (224). Similar phenotypes are observed in indirect ''IGF2'' overexpression in ''PEPCK''-''GH'' transgenic mice that overexpress ''GH'' (225). However, simple overexpression of ''IGF2'' was insufficient to initiate adrenocortical tumorigenesis. Perturbation of the ''IGF2'' locus, with upregulation of maternally imprinted genes (''IGF2''), and downregulation | |||
of paternally imprinted genes (''H19'' and ''CDKN1C''), is frequently observed in ACCs (226). However, 11p15 LOH has been shown to be a stronger predictor for shorter disease- free survival than simple levels of ''IGF2'' overexpression (184). Based on this observation, it is hypothesized that additional genetic changes, such as loss of maternally expressed ''CDKN1C'' and ''H19'', may contribute to adrenal tumorigenesis (184). The findings of high ''IGF2'' expression levels and the knowledge of an increased incidence of ACC in BWS led to the investigation of IGF-1R as a therapeutic target. In an NCI-H295 xenograft mouse model, IGF pathway inhibition by the small-molecule inhibitor NVP-AEW541 and the monoclonal IGF-1R antibody IMCA12 showed an antitumor effect. Furthermore, the combined treatment of NCI-H295 cells with IGF-1R antagonists and mitotane resulted in a synergistic antiproliferative effect in vitro and in vivo in tumor xenografts (223, 227). | |||
==Gross Pathology== | ==Gross Pathology== | ||
Revision as of 19:46, 20 September 2017
|
Adrenocortical carcinoma Microchapters |
|
Differentiating Adrenocortical carcinoma from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Study |
|
Adrenocortical carcinoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Adrenocortical carcinoma pathophysiology |
|
Risk calculators and risk factors for Adrenocortical carcinoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2]Shivali Marketkar, M.B.B.S. [3]Ahmad Al Maradni, M.D. [4]
Overview
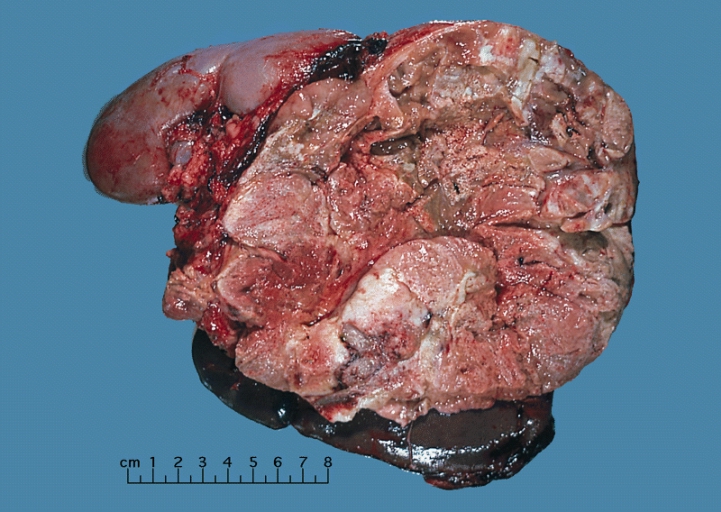
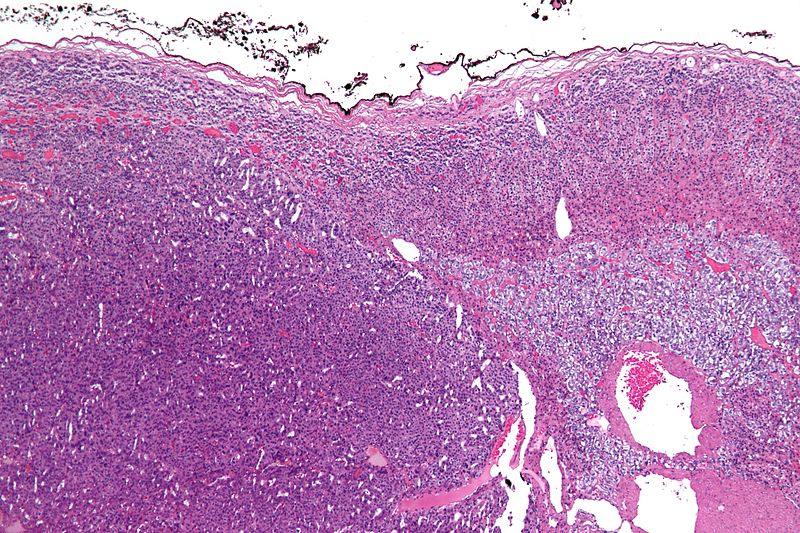
On gross pathology, a large tan-yellow surface with areas of hemorrhage and necrosis is a characteristic finding of adrenocortical carcinoma. On microscopic histopathological analysis, sheets of atypical cells with some resemblance to the cells of the normal adrenal cortex are a characteristic finding of adrenocortical carcinoma.
Pathophysiology
ACCs are typically large tumors upon clinical presentation, often measuring more than 6 cm in diameter (97). They are bilateral in 2% to 10% of cases (98, 99). Metastases to the liver, lungs, or lymph nodes can be seen, and invasion of adjacent organs or venous extension into the renal vein and/or inferior vena cava may be present. Inferior vena cava invasion has been reported in 9% to 19% of cases at presentation (98). Due to the presence of internal hemorrhage, necrosis, and calcifications, these tumors tend to vary in appearance with frequent heterogeneous enhancement.
Genetics
The genetic dissection of ACC has revealed genomic aberrations that contribute to neoplastic transformation of adrenocortical cells:
1. Clonality
- ACCs initiate from monoclonal cell populations, suggesting that mutation events lead to clonal expansion and ultimate progression to cancer. (177, 178).
- Flow cytometry revealed aneuploidy in ACC.(180).aneuploidy was observed in 75% of ACC.
- Assessment of aneuploidy with histopathological criteria in 7 of 9 adrenal tumors revealed a high correlation with Weiss score >3 (indicative of malignancy) (182).
- No significant difference in overall survival was observed in patients with ACC exhibiting aneuploidy vs patients with ACC exhibiting diploid neoplasms. (126).
- An initial study identified elevated expression of genes involved in cell proliferation in ACC, such as IGF2, compared with increased expression of steroidogenic genes in ACA. (190).
- Giordano et al identified unique transcriptionally activated (12q and 5q) and repressed (11q, 1p, and 17p) chromosomal regions in 33 ACCs vs 22 ACAs in a microarray study.(192)
- Giordano et al (192) determined that ACC with high histological grade exhibited overexpression of cell cycle and functional aneuploidy genes and leading to the decreased survival of patients.
3. MicroRNAs
- MicroRNAs are RNAs that are important in the regulation of gene expression.
- Numerous miRNAs have been identified in the regulation of various cellular processes such as proliferation, apoptosis, and differentiation. (194).
- Dysregulation of miRNAs, such as overexpression or deletion, plays an important role in diseases.
- Mistargeting of the miRNAs, resulting in inhibition or activation of various oncogenes, tumor suppressors, and other factors important in tumor angiogenesis. (196).
- The investigation identified 14 upregulated miRNAs and 9 downregulated miRNAs unique to ACC. (198).
- Upregulated miRNAs in ACCs included miR-184, miR-210, and miR-503.
- Downregulated miRNAs included miR-214, miR-375, and miR-511. (197).
- Levels of miR-184, miR-503, and miR-511 are able to distinguish benign from malignant adrenal tumors. (197).
- MiR-483 was found to be significantly upregulated in pediatric ACCs.
- MiR-99a and miR-100 are bioinformatically predicted to target the 3- untranslated regions of IGF1R, RPTOR, and FRAP1 and were experimentally confirmed to target several components of the IGF-1 signaling pathway. (199)
4. Gene mutations
- Targeted genetic analyses have identified somatic genetic changes in TP53, MEN1, IGF2, IGF2R, and p16.
- TP53 located on 17p13 is the most commonly mutated gene in ACC, present in at least one-third ofACCs (140, 142, 202) (203).
- LOH in the gene encoding p16ink/p14arf, CDKN2A is observed in a subset of ACCs. The tumor suppressor function of this gene has been established in multiple cancers (204). LOH of 11q13 has been identified in 83% of samples. (185)
- MEN1 (located on 11q13) somatic mutations are unusual in sporadic ACC.
- The canonical Wnt pathway, the catenin gene, and CTNNB1 have been identified as activating point mutations in over 25% of both ACAs and ACCs in children and adults. (149, 206–208).
5. Chromosomal aberrations
- Comparative genomic hybridization(CGH) can identify structural chromosomal abnormalities within ACCs.
- ACCs showed complex chromosomal alterations. ACCs contained multiple chromosomal gains or losses with a mean of 10 events.
- The newest study confirmed increased alterations in ACC (44%) compared with ACAs (10%). 188
- In ACCs, the frequently observed chromosomal gains at 5, 7, 12, 16, 19, and 20 and losses at 13 and 22 were confirmed.
- The group identified genes within these regions with potential tumorigenic potential including fibroblast growth factor 4 (FGF4), cyclin-dependent kinase 4 (CDK4), and cyclin E1 (CCNE1). The study confirmed the diagnostic utility of 6 loci (5q, 7p, 11p, 13q, 16q, and 22q) in the differentiation of ACA and ACC(188).
- Genomic aberration at chromosomes 5, 12, and 17 are predicted to illustrate genes that initiate or maintain neoplastic transformation. Chromosome 17, specifically at 17p13, contains the well-known tumor suppressor gene TP53.
6. Epigenetic changes
- DNA methylation involves the addition of a methyl group to the cytosine pyrimidine ring or adenine purine ring.
- Dysregulation in this process is observed in tumor cells.
- A recent study revealed hypermethylation of promoters in ACCs with correlation to poor survival and identified H19, PLAGL1, G0S2, and NDRG2 as silenced genes also provided evidence about the role of methylation in ACC tumorigenesis, particularly in the 11p15 locus containing IGF2 and H19.
The IGF signaling pathway consists of ligands (IGF-1 and IGF-2), receptors (IGF-1 receptor [IGF-1R], IGF-2R, and insulin receptor), IGF binding proteins 1–6, and IGF binding protein proteases.
The binding of the mitogenic polypeptides to their receptors activates the downstream AKT/PI3K and MAPK pathways to regulate cellular processes of metabolism, differentiation, proliferation, and apoptosis.
The IGF pathway mediates ACTH-induced prenatal adrenal growth, fetal and adult steroidogenesis, and organ maintenance (209–212).
In the developing fetal organ, IGF1 expression is restricted to the capsule, whereas IGF2 expression is enriched in the cortex (213).
In the adult adrenal cortex, both IGF-1 and IGF-2 stimulate basal and ACTH-induced steroidogenesis (210, 214). Overall, the main role of IGF-2 lies in fetal development and growth, whereas IGF-1 acts mainly postnatally. Prominent overexpression of IGF2 and alterations of the IGF2/H19 locus have been identified in sporadic ACC(174, 190, 215). The IGF2 gene is located on 11p15, which also includes a noncoding H19 gene and a cyclindependent kinase inhibitor, CDKN1C (p57KIP2) (216, 217), and 80% to 90% of all ACCs show very high IGF2 expression (_100-fold over normal and ACA) (174, 218– 220). Interestingly, relative expression of Igf2 is much higher than in tissues from mice resembling human BWS, in which genetic changes result in an _2-fold upregulation. High IGF2 expression levels in adrenal tumors, when analyzing malignant and benign tumors, are associated with a 5-fold increased risk for recurrence and a shorter disease-free survival (184, 191). Pediatric ACCs reveal an _20-fold overexpression of IGF2. Various cell culture studies using ACC cell lines suggest a paracrine or autocrine effect of IGF-2 and mitogenic activity through IGF-1R (156, 221–223). PEPCK-IGF2 transgenic mice that overexpress IGF2 have adrenocortical hyperplasia and enhanced steroidogenesis (224). Similar phenotypes are observed in indirect IGF2 overexpression in PEPCK-GH transgenic mice that overexpress GH (225). However, simple overexpression of IGF2 was insufficient to initiate adrenocortical tumorigenesis. Perturbation of the IGF2 locus, with upregulation of maternally imprinted genes (IGF2), and downregulation
of paternally imprinted genes (H19 and CDKN1C), is frequently observed in ACCs (226). However, 11p15 LOH has been shown to be a stronger predictor for shorter disease- free survival than simple levels of IGF2 overexpression (184). Based on this observation, it is hypothesized that additional genetic changes, such as loss of maternally expressed CDKN1C and H19, may contribute to adrenal tumorigenesis (184). The findings of high IGF2 expression levels and the knowledge of an increased incidence of ACC in BWS led to the investigation of IGF-1R as a therapeutic target. In an NCI-H295 xenograft mouse model, IGF pathway inhibition by the small-molecule inhibitor NVP-AEW541 and the monoclonal IGF-1R antibody IMCA12 showed an antitumor effect. Furthermore, the combined treatment of NCI-H295 cells with IGF-1R antagonists and mitotane resulted in a synergistic antiproliferative effect in vitro and in vivo in tumor xenografts (223, 227).
Gross Pathology
On gross pathology, adrenocortical carcinomas are often large, with a tan-yellow cut surface and areas of hemorrhage and necrosis.
Shown above is a large adrenal cortical carcinoma resected from a 27-year-old woman. The tumor measured 17 cm in diameter and invaded kidney and spleen which necessitated en bloc removal of these organs with tumor. Patient had evidence of virilization.
Microscopic Pathology
On microscopic examination, the tumor usually displays sheets of atypical cells with some resemblance to the cells of the normal adrenal cortex. The presence of invasion and mitotic activity helps differentiate small cancers from adrenocortical adenomas.[1]
The Weiss criteria of adrenocortical malignancy comprise the most reliable histopathological scoring system differentiating ACC from ACA 9–11
ACC can be diagnosed by the presence of at least 3 of the 9 Weiss criteria:
- Three relate to cytological features (nuclear grade, mitoses and atypical mitoses)
- Three refer to tumor structure (clear cells, diffuse architecture, and confluent necrosis)
- Three relate to invasion (venous invasion, sinusoidal invasion, and capsular infiltration)
Micrograph of an adrenocortical carcinoma (left of image - dark blue) and the adrenal cortex it arose from (right-top of image - pink/light blue). Benign adrenal medulla is present (right-middle of image - gray/blue). H&E stain.
Video
Shown below is a video explaining the histology of adrenocortical carcinoma
{{#ev:youtube|7jMFENhPaOM}}
References
- ↑ Richard Cote, Saul Suster, Lawrence Weiss, Noel Weidner (Editor). Modern Surgical Pathology (2 Volume Set). London: W B Saunders. ISBN 0-7216-7253-1.