Acute respiratory distress syndrome pathophysiology: Difference between revisions
mNo edit summary |
|||
| Line 6: | Line 6: | ||
==Pathophysiology== | ==Pathophysiology== | ||
ARDS typically develops within 24 to 48 hours of the provoking illness or injury and is classically divided into three phases:<br> | |||
* | *'''Exudative phase (''within 24-48 hours'')''': Systemic inflammation results in increased alveolar capillary permeability and leads to the formation of hyaline membranes along alveolar walls, accumulation of proteinaceous exudate within the alveolar air spaces (''non-cardiogenic pulmonary edema''), and extravasation of inflammatory cells (predominantly [[neutrophils|neutrophils]] and [[macrophages|macrophages]]) into the lung parenchyma, leading to extensive alveolar damage and sometimes hemorrhage into alveoli | ||
*'''Proliferative phase (''within 5-7 days'')''': Fibroblast proliferation, collagen deposition, and early fibrotic changes are observed within the pulmonary interstitium as alveolar exudate and hyaline membranes begin to be absorbed | |||
*'''Fibrotic phase (''within several weeks'')''': Most patients with ARDS will develop some degree of pulmonary fibrosis, of which at least one-quarter will go on to develop a restrictive ventilatory defect on pulmonary function tests<ref name="pmid23520315">{{cite journal| author=Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP| title=The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. | journal=Eur Respir J | year= 2014 | volume= 43 | issue= 1 | pages= 276-85 | pmid=23520315 | doi=10.1183/09031936.00196412 | pmc=4015132 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23520315 }} </ref>; the development and extent of pulmonary fibrosis in ARDS correlates with an increased mortality risk<ref name="pmid7813276">{{cite journal| author=Martin C, Papazian L, Payan MJ, Saux P, Gouin F| title=Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. | journal=Chest | year= 1995 | volume= 107 | issue= 1 | pages= 196-200 | pmid=7813276 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7813276 }} </ref> | |||
* | |||
* | |||
=== | ===Genetic Susceptibility=== | ||
The role of genetics in the development of ARDS is an ongoing area of research. While studies have demonstrated associations between certain genetic factors (including [[single-nucleotide polymorphisms|single-nucleotide polymorphisms]] and allelic variants of [[angiotensin-converting enzyme|angiotensin-converting enzyme]]<ref name="pmid16484896">{{cite journal| author=Jerng JS, Yu CJ, Wang HC, Chen KY, Cheng SL, Yang PC| title=Polymorphism of the angiotensin-converting enzyme gene affects the outcome of acute respiratory distress syndrome. | journal=Crit Care Med | year= 2006 | volume= 34 | issue= 4 | pages= 1001-6 | pmid=16484896 | doi=10.1097/01.CCM.0000206107.92476.39 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16484896 }} </ref><sup>,</sup><ref name="pmid23364437">{{cite journal| author=Cardinal-Fernández P, Ferruelo A, El-Assar M, Santiago C, Gómez-Gallego F, Martín-Pellicer A et al.| title=Genetic predisposition to acute respiratory distress syndrome in patients with severe sepsis. | journal=Shock | year= 2013 | volume= 39 | issue= 3 | pages= 255-60 | pmid=23364437 | doi=10.1097/SHK.0b013e3182866ff9 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23364437 }} </ref>) and increased susceptibility to developing ARDS, the nature and implications of these relationships remain uncertain.<ref name="pmid23048207">{{cite journal| author=Tejera P, Meyer NJ, Chen F, Feng R, Zhao Y, O'Mahony DS et al.| title=Distinct and replicable genetic risk factors for acute respiratory distress syndrome of pulmonary or extrapulmonary origin. | journal=J Med Genet | year= 2012 | volume= 49 | issue= 11 | pages= 671-80 | pmid=23048207 | doi=10.1136/jmedgenet-2012-100972 | pmc=3654537 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23048207 }} </ref> | |||
===Pathology=== | |||
*On gross pathology, the lungs are firm, boggy, and dusky, and they typically weigh more than healthy lungs due to edema | |||
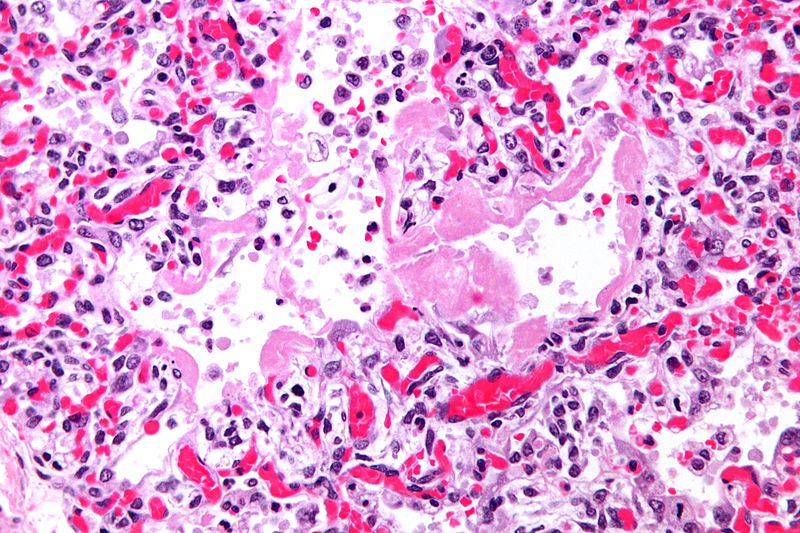
*On microscopic histopathological analysis, the lung parenchyma demonstrates [http://www.wikidoc.org/index.php/File:Hyaline_membranes_-_very_high_mag.jpg hyaline membranes] lining the alveolar air spaces, edema fluid within alveoli and the interstitium, shedding of type I pneumocytes and proliferation of type II pneumocytes, infiltration of polymorphonuclear and other inflammatory cells into the interstitial and alveolar compartments, thrombosis and obliteration of pulmonary capillaries, and occasionally hemorrhage into alveoli | |||
:*Features specific to the underlying disease process (e.g., [[pneumonia|bacterial pneumonia]] or [[aspiration pneumonitis|aspiration pneumonitis]]) are often seen as well | |||
:*As ARDS progresses, alveolar infiltrates are reabsorbed and the inflammatory milieu is replaced by increased collagen deposition and proliferating fibroblasts, culminating in interstitial fibrosis | |||
[[File:Hyaline membranes - very high mag.jpg|thumb|Hyaline membranes - very high magnification]] | |||
* | |||
* | |||
* | |||
==References== | ==References== | ||
Revision as of 05:00, 20 June 2016
|
Acute respiratory distress syndrome Microchapters |
|
Differentiating Acute respiratory distress syndrome from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Acute respiratory distress syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Acute respiratory distress syndrome pathophysiology |
|
Acute respiratory distress syndrome pathophysiology in the news |
|
Blogs on Acute respiratory distress syndrome pathophysiology |
|
Directions to Hospitals Treating Acute respiratory distress syndrome |
|
Risk calculators and risk factors for Acute respiratory distress syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Acute respiratory distress syndrome primarily results from the diffuse inflammation of lung parenchyma. Loss of aeration can cause fundamental changes in inflammation amplification and progression.
Pathophysiology
ARDS typically develops within 24 to 48 hours of the provoking illness or injury and is classically divided into three phases:
- Exudative phase (within 24-48 hours): Systemic inflammation results in increased alveolar capillary permeability and leads to the formation of hyaline membranes along alveolar walls, accumulation of proteinaceous exudate within the alveolar air spaces (non-cardiogenic pulmonary edema), and extravasation of inflammatory cells (predominantly neutrophils and macrophages) into the lung parenchyma, leading to extensive alveolar damage and sometimes hemorrhage into alveoli
- Proliferative phase (within 5-7 days): Fibroblast proliferation, collagen deposition, and early fibrotic changes are observed within the pulmonary interstitium as alveolar exudate and hyaline membranes begin to be absorbed
- Fibrotic phase (within several weeks): Most patients with ARDS will develop some degree of pulmonary fibrosis, of which at least one-quarter will go on to develop a restrictive ventilatory defect on pulmonary function tests[1]; the development and extent of pulmonary fibrosis in ARDS correlates with an increased mortality risk[2]
Genetic Susceptibility
The role of genetics in the development of ARDS is an ongoing area of research. While studies have demonstrated associations between certain genetic factors (including single-nucleotide polymorphisms and allelic variants of angiotensin-converting enzyme[3],[4]) and increased susceptibility to developing ARDS, the nature and implications of these relationships remain uncertain.[5]
Pathology
- On gross pathology, the lungs are firm, boggy, and dusky, and they typically weigh more than healthy lungs due to edema
- On microscopic histopathological analysis, the lung parenchyma demonstrates hyaline membranes lining the alveolar air spaces, edema fluid within alveoli and the interstitium, shedding of type I pneumocytes and proliferation of type II pneumocytes, infiltration of polymorphonuclear and other inflammatory cells into the interstitial and alveolar compartments, thrombosis and obliteration of pulmonary capillaries, and occasionally hemorrhage into alveoli
- Features specific to the underlying disease process (e.g., bacterial pneumonia or aspiration pneumonitis) are often seen as well
- As ARDS progresses, alveolar infiltrates are reabsorbed and the inflammatory milieu is replaced by increased collagen deposition and proliferating fibroblasts, culminating in interstitial fibrosis
References
- ↑ Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP (2014). "The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance". Eur Respir J. 43 (1): 276–85. doi:10.1183/09031936.00196412. PMC 4015132. PMID 23520315.
- ↑ Martin C, Papazian L, Payan MJ, Saux P, Gouin F (1995). "Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients". Chest. 107 (1): 196–200. PMID 7813276.
- ↑ Jerng JS, Yu CJ, Wang HC, Chen KY, Cheng SL, Yang PC (2006). "Polymorphism of the angiotensin-converting enzyme gene affects the outcome of acute respiratory distress syndrome". Crit Care Med. 34 (4): 1001–6. doi:10.1097/01.CCM.0000206107.92476.39. PMID 16484896.
- ↑ Cardinal-Fernández P, Ferruelo A, El-Assar M, Santiago C, Gómez-Gallego F, Martín-Pellicer A; et al. (2013). "Genetic predisposition to acute respiratory distress syndrome in patients with severe sepsis". Shock. 39 (3): 255–60. doi:10.1097/SHK.0b013e3182866ff9. PMID 23364437.
- ↑ Tejera P, Meyer NJ, Chen F, Feng R, Zhao Y, O'Mahony DS; et al. (2012). "Distinct and replicable genetic risk factors for acute respiratory distress syndrome of pulmonary or extrapulmonary origin". J Med Genet. 49 (11): 671–80. doi:10.1136/jmedgenet-2012-100972. PMC 3654537. PMID 23048207.