21-hydroxylase deficiency pathophysiology: Difference between revisions
No edit summary |
Aditya Ganti (talk | contribs) |
||
| (32 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{ | {{21-hydroxylase deficiency}} | ||
{{CMG}}; '''Associate Editor-In-Chief:''' {{MJ}} | {{CMG}}; '''Associate Editor-In-Chief:''' {{MJ}}, {{AAM}} | ||
==Overview== | ==Overview== | ||
The progression to 21-hydroxylase deficiency usually involves the defective conversion of [[17-hydroxyprogesterone]] to [[11-deoxycorticosterone|11-deoxycortisol]] which results in decreased [[cortisol]] synthesis and therefore increased [[corticotropin]] ([[ACTH|ACTH)]] secretion. The resulting [[adrenal]] stimulation leads to increased production of [[androgens]] due to shunting of the pathway to [[androgen]] synthesis. More than 95% of cases of [[congenital adrenal hyperplasia]] ([[Congenital adrenal hyperplasia|CAH]]) are caused by 21-hydroxylase deficiency. The clinical manifestations of [[congenital adrenal hyperplasia]] is closely related to the type and severity of disease. The severity of disease relates to the type of [[mutation]] which causes [[enzyme]] inactivity or hypo-activity. There is a lack of [[enzyme]] in classic form of 21-hydroxylase deficiency; while in the non-classic form, [[enzymatic]] activity is reduced but sufficient to maintain normal [[glucocorticoid]] and [[mineralocorticoid]] production. The [[gene]] responsible for 21-hydroxylase deficiency is [[CYP21A1|CYP21A]]. This [[gene]] is located within the [[Human leukocyte antigen|human leucocyte antigen]] class III region of [[chromosome 6]]. Meiotic [[recombination]] occurs in this genomic region as a result of the high degree of [[sequence homology]] between [[CYP21A2]] and its [[pseudogene]] [[CYP21A1]]. Approximately 70% of [[CYP21A2]] [[genetic mutation]] is due to [[gene conversion]] and [[Microdeletion|micro-deletions]] in [[CYP21A1]] [[gene]]. | |||
== Pathophysiology == | == Pathophysiology == | ||
In patients with 21-hydroxylase deficiency, there is a defective conversion of 17-hydroxyprogesterone to 11-deoxycortisol which results in decreased cortisol synthesis and therefore increased corticotropin (ACTH) secretion | ===Pathogenesis=== | ||
* [[21-hydroxylase]] enzyme is involved in the synthesis of [[aldosterone]] in [[zona glomerulosa]] and [[cortisol]] in [[zona fasciculata]]. Lack of 21-hydroxylase enzyme leads to decrease in [[cortisol]] and [[aldosterone]] levels and the rest of synthesis pathways produce extra [[androgens]] and lead to [[hirsutism]]. | |||
* More than 95% of all cases of [[congenital adrenal hyperplasia]] ([[CAH]]) are caused by 21-hydroxylase deficiency; the clinical manifestations of 21-hydroxylase deficiency is closely related to the type and severity of disease. | |||
* The severity of disease relates to the type of [[mutation]], which causes [[enzyme]] inactivity or hypo activity. | |||
* There is a lack of [[enzyme]] in classic type of [[21-hydroxylase]] deficiency; while in the non-classic form, enzymatic activity is reduced but sufficient to maintain normal [[glucocorticoid]] and [[mineralocorticoid]] production. | |||
===Glucocorticoid pathway=== | |||
* In patients with 21-hydroxylase deficiency in [[zona fasciculata]], there is a defective conversion of [[17-hydroxyprogesterone]] to 11-[[deoxycortisol]] which results in decreased [[cortisol]] synthesis and therefore increased [[Corticotropin|corticotropin (ACTH)]] secretion. | |||
===Mineralocorticoids pathway=== | |||
* In patients with 21-hydroxylase deficiency in [[zona glomerulosa]], there is a defective conversion of [[progesterone]] to 11-deoxycortisterone which results in decreased [[aldosterone]] synthesis. | |||
* The lack of [[aldosterone]] causes large amounts of [[sodium]] loss in the [[urine]]. Urinary [[sodium]] concentrations are more than 50 mEq/L. As a result of high amount of [[sodium]] loss, [[blood volume]] and [[blood pressure]] can not be maintained in normal ranges. | |||
* Due to [[mineralocorticoid]] deficiency, [[potassium]] and [[acid]] excretion are also impaired resulting in [[hyperkalemia]] and [[metabolic acidosis]]. | |||
* There is significant water loss and symptoms of [[dehydration]] due to salt wasting within the first two week of life. In severe form of [[CAH]], [[vomiting]], severe [[dehydration]], circulatory collapse and [[shock]]<nowiki/>develops in the second or third week of life. | |||
[[ | ===Androgen pathway=== | ||
* In the [[androgen]] synthesis pathway, 21-hydroxylase enzyme does not have a direct role; therefore with extra amount of other products from blocked [[cortisol]] and [[aldosterone]] synthesis, [[androgen]] pathway have extra [[Precursors|precursor]] [[metabolites]] resulting in [[androgen]] excess in the form of [[dehydroepiandrosterone]] and [[androstenedione]] accumulation. | |||
* On the other hand, lack of [[cortisol]] removes the negative feedback on the [[pituitary gland]], resulting in an increase in [[ACTH]] level and consequently more increase in [[androgen]] synthesis pathway. High [[androgen]] level in [[21-hydroxylase]] deficient women during [[pregnancy]] causes [[ambiguous genitalia]] in female fetus; also in milder forms induces [[hirsutism]] and [[virilization]] in women. [[Adrenal]] [[androgens]] produce little effect on the [[genitalia]] of male [[infants]] with severe [[CAH]]. Excess [[androgen]] can cause [[precocious puberty]] in male child. | |||
Below is the [[hormonal]] pathway of [[adrenal]] [[steroids]] and related [[enzymes]], also the mechanism of 21 hydroxylase deficiency symptoms.<ref name="pmid10857554">{{cite journal |vauthors=White PC, Speiser PW |title=Congenital adrenal hyperplasia due to 21-hydroxylase deficiency |journal=Endocr. Rev. |volume=21 |issue=3 |pages=245–91 |year=2000 |pmid=10857554 |doi=10.1210/edrv.21.3.0398 |url=}}</ref><ref name="pmid20823466">{{cite journal |vauthors=Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC |title=Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline |journal=J. Clin. Endocrinol. Metab. |volume=95 |issue=9 |pages=4133–60 |year=2010 |pmid=20823466 |pmc=2936060 |doi=10.1210/jc.2009-2631 |url=}}</ref> | |||
[[image:21 hydroxylase.gif|center|frame|800px|Adrenal steroid synthesis pathways in adrenal cortex and related enzymes <ref name="urlFile:Adrenal Steroids Pathways.svg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/wiki/File:Adrenal_Steroids_Pathways.svg|title=File:Adrenal Steroids Pathways.svg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
== Genetics == | == Genetics == | ||
* Congenital adrenal hyperplasia subtypes are all autosomal recessive and monogenetic. The disease manifestation follows the allele that results in a more functional enzyme, and generally correlation between genotype and phenotype is good.<ref name="pmid20926536">{{cite journal |vauthors=Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP |title=Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency |journal=J. Clin. Endocrinol. Metab. |volume=96 |issue=1 |pages=E161–72 |year=2011 |pmid=20926536 |pmc=3038490 |doi=10.1210/jc.2010-0319 |url=}}</ref><ref name="pmid23359698">{{cite journal |vauthors=New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, Sun L, Zaidi M, Wilson RC, Yuen T |title=Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=110 |issue=7 |pages=2611–6 |year=2013 |pmid=23359698 |pmc=3574953 |doi=10.1073/pnas.1300057110 |url=}}</ref> | * [[Congenital adrenal hyperplasia]] subtypes are all [[autosomal recessive]] and [[Monogenic disorder|monogenetic]]. The disease manifestation follows the [[allele]] that results in a more functional enzyme, and generally correlation between [[genotype]] and [[phenotype]] is good.<ref name="pmid20926536">{{cite journal |vauthors=Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP |title=Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency |journal=J. Clin. Endocrinol. Metab. |volume=96 |issue=1 |pages=E161–72 |year=2011 |pmid=20926536 |pmc=3038490 |doi=10.1210/jc.2010-0319 |url=}}</ref><ref name="pmid23359698">{{cite journal |vauthors=New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, Sun L, Zaidi M, Wilson RC, Yuen T |title=Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=110 |issue=7 |pages=2611–6 |year=2013 |pmid=23359698 |pmc=3574953 |doi=10.1073/pnas.1300057110 |url=}}</ref> | ||
=== CYP21A gene === | |||
* The [[gene]] responsible for 21-hydroxylase deficiency is [[CYP21A1|CYP21A]]. This gene is located within the [[Human leukocyte antigen|human leucocyte antigen]] class III region of [[chromosome 6]]. | |||
[[CYP21A1|CYP21A]] gene has two types: | |||
===== CYP21A2 ===== | |||
* An active [[gene]] called [[CYP21A2]], which encodes 21-hydroxylase, a [[cytochrome P450]] type II [[enzyme]] containing 495 [[amino acids]]. | |||
* | ===== CYP21A1 ===== | ||
* This [[gene]] is a non-functional [[pseudogene]] named [[CYP21A1]] or CYP21P. This [[pseudogene]] produces an [[enzyme]] with no activity because it lacks eight bases from [[codons]] 110-112, which results in a [[stop codon]].<ref name="pmid3487786">{{cite journal |vauthors=White PC, New MI, Dupont B |title=Structure of human steroid 21-hydroxylase genes |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=83 |issue=14 |pages=5111–5 |year=1986 |pmid=3487786 |pmc=323900 |doi= |url=}}</ref> | |||
* Meiotic recombination events occurs in this genomic region as a result of the high degree of sequence homology between CYP21A2 and its pseudogene CYP21A1. | ===== Mutation mechanisms: ===== | ||
** Approximately 70% of CYP21A2 | * Meiotic [[recombination]] events occurs in this [[genomic]] region as a result of the high degree of [[sequence homology]] between [[CYP21A2]] and its [[pseudogene]] [[CYP21A1]]. | ||
** Approximately 25% to 30% are chimeric genes due to large deletions. | ** Approximately 70% of disease associated with [[CYP21A2]] is due to [[gene conversion]] and [[Microdeletion|microdeletions]] in [[CYP21A1]] [[gene]]. | ||
** Approximately 1% to 2% of cases are due to de novo mutations because of high variability of the CYP21A2 locus. | ** Approximately 25% to 30% are [[Chimerism|chimeric]] [[genes]] due to large [[Deletion (genetics)|deletions]]. | ||
** Chromosome 6 uniparental disomy is rare cause of 21-hydroxylase deficiency with an unknown prevalence. | ** Approximately 1% to 2% of cases are due to [[De novo mutation|de novo mutations]] because of high variability of the [[CYP21A2]] [[locus]]. | ||
** [[Chromosome 6]] [[uniparental disomy]] is rare cause of [[21-hydroxylase]] deficiency with an unknown [[prevalence]]. | |||
* [[Gene]] [[mutations]] that completely inactivates [[CYP21A2]] [[gene]] will result in the classic type and salt-wasting subtype. | |||
* [[Gene]] [[mutations]] that maintain 1–2% of 21-hydroxylase activity will result in classic type and non-salt-wasting subtype. These patients have minimal [[aldosterone]] production that prevents a [[neonatal]] [[adrenal crisis]].<ref name="pmid2831244">{{cite journal |vauthors=Fiet J, Gueux B, Gourmelen M, Kuttenn F, Vexiau P, Couillin P, Pham-Huu-Trung MT, Villette JM, Raux-Demay MC, Galons H |title=Comparison of basal and adrenocorticotropin-stimulated plasma 21-deoxycortisol and 17-hydroxyprogesterone values as biological markers of late-onset adrenal hyperplasia |journal=J. Clin. Endocrinol. Metab. |volume=66 |issue=4 |pages=659–67 |year=1988 |pmid=2831244 |doi=10.1210/jcem-66-4-659 |url=}}</ref> | |||
==Gross Pathology== | |||
[[Gross]] [[pathology]] findings in patients with 21 hydroxylase deficiency are:<ref name="radio">Congenital adrenal hyperplasia. Dr Henry Knipe and Dr M Venkatesh . Radiopaedia.org 2015.http://radiopaedia.org/articles/congenital-adrenal-hyperplasia</ref><ref name="pmid25372578">{{cite journal |vauthors=Teixeira SR, Elias PC, Andrade MT, Melo AF, Elias Junior J |title=The role of imaging in congenital adrenal hyperplasia |journal=Arq Bras Endocrinol Metabol |volume=58 |issue=7 |pages=701–8 |year=2014 |pmid=25372578 |doi= |url=}}</ref> | |||
*Enlarged [[adrenal glands]] | |||
*Wrinkled surface of [[adrenal glands]] | |||
*Cerebriform pattern in [[adrenal glands]] ([[pathognomonic]] sign) | |||
*Normal [[ultrasound]] appearance | |||
==Microscopic Pathology== | |||
In [[21-hydroxylase]] deficiency [[microscopic]] findings may include: | |||
* Diffuse [[Adrenal cortex|cortical]] [[hyperplasia]] with smaller [[Cell (biology)|cells]] | |||
* The [[Cell (biology)|cell]] [[cytoplasm]] can be [[Vacuolization|vacuolated]], and often more [[basophilic]]. | |||
* Rare [[mitotic]] figures may be present | |||
* The [[hyperplastic]] [[Cell (biology)|cells]] typically lack features of [[atypia|cellular atypia]].<ref name="urlAdrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas">{{cite web |url=https://ntp.niehs.nih.gov/nnl/endocrine/adrenal/hyperpl/index.htm |title=Adrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas |format= |work= |accessdate=}}</ref> | |||
{| | |||
| | |||
[[Image:Cah mic.jpg|thumb|200px|frame|Adrenal gland, Cortex - Hyperplasia in a female rat from a chronic study. There is a hyperplastic lesion (H) in which cortical cells are increased in number but are smaller in size than adjacent normal cortical cells (NC)<ref name="urlAdrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas">{{cite web |url=https://ntp.niehs.nih.gov/nnl/endocrine/adrenal/hyperpl/index.htm |title=Adrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas |format= |work= |accessdate=}}</ref>]] | |||
| | |||
[[Image:Cah.jpg|thumb|250px|frame|Adrenal gland, Cortex - Hyperplasia in a male rat from a chronic study. There are two adjacent foci of hyperplasia (H) in the zona fasciculata.<ref name="urlAdrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas">{{cite web |url=https://ntp.niehs.nih.gov/nnl/endocrine/adrenal/hyperpl/index.htm |title=Adrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas |format= |work= |accessdate=}}</ref>]] | |||
|} | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
{{WH}} | |||
{{WS}} | |||
[[Category:Disease]] | |||
[[Category:Pediatrics]] | |||
[[Category:Endocrinology]] | |||
[[Category:Genetic disorders]] | |||
[[Category:Intersexuality]] | |||
[[Category:Medicine]] | |||
[[Category: Up-To-Date]] | |||
| |||
Latest revision as of 15:39, 24 July 2020
|
21-hydroxylase deficiency Microchapters |
|
Differentiating 21-Hydroxylase Deficiency from other Diseases |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
21-hydroxylase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of 21-hydroxylase deficiency pathophysiology |
|
Risk calculators and risk factors for 21-hydroxylase deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Mehrian Jafarizade, M.D [2], Ahmad Al Maradni, M.D. [3]
Overview
The progression to 21-hydroxylase deficiency usually involves the defective conversion of 17-hydroxyprogesterone to 11-deoxycortisol which results in decreased cortisol synthesis and therefore increased corticotropin (ACTH) secretion. The resulting adrenal stimulation leads to increased production of androgens due to shunting of the pathway to androgen synthesis. More than 95% of cases of congenital adrenal hyperplasia (CAH) are caused by 21-hydroxylase deficiency. The clinical manifestations of congenital adrenal hyperplasia is closely related to the type and severity of disease. The severity of disease relates to the type of mutation which causes enzyme inactivity or hypo-activity. There is a lack of enzyme in classic form of 21-hydroxylase deficiency; while in the non-classic form, enzymatic activity is reduced but sufficient to maintain normal glucocorticoid and mineralocorticoid production. The gene responsible for 21-hydroxylase deficiency is CYP21A. This gene is located within the human leucocyte antigen class III region of chromosome 6. Meiotic recombination occurs in this genomic region as a result of the high degree of sequence homology between CYP21A2 and its pseudogene CYP21A1. Approximately 70% of CYP21A2 genetic mutation is due to gene conversion and micro-deletions in CYP21A1 gene.
Pathophysiology
Pathogenesis
- 21-hydroxylase enzyme is involved in the synthesis of aldosterone in zona glomerulosa and cortisol in zona fasciculata. Lack of 21-hydroxylase enzyme leads to decrease in cortisol and aldosterone levels and the rest of synthesis pathways produce extra androgens and lead to hirsutism.
- More than 95% of all cases of congenital adrenal hyperplasia (CAH) are caused by 21-hydroxylase deficiency; the clinical manifestations of 21-hydroxylase deficiency is closely related to the type and severity of disease.
- The severity of disease relates to the type of mutation, which causes enzyme inactivity or hypo activity.
- There is a lack of enzyme in classic type of 21-hydroxylase deficiency; while in the non-classic form, enzymatic activity is reduced but sufficient to maintain normal glucocorticoid and mineralocorticoid production.
Glucocorticoid pathway
- In patients with 21-hydroxylase deficiency in zona fasciculata, there is a defective conversion of 17-hydroxyprogesterone to 11-deoxycortisol which results in decreased cortisol synthesis and therefore increased corticotropin (ACTH) secretion.
Mineralocorticoids pathway
- In patients with 21-hydroxylase deficiency in zona glomerulosa, there is a defective conversion of progesterone to 11-deoxycortisterone which results in decreased aldosterone synthesis.
- The lack of aldosterone causes large amounts of sodium loss in the urine. Urinary sodium concentrations are more than 50 mEq/L. As a result of high amount of sodium loss, blood volume and blood pressure can not be maintained in normal ranges.
- Due to mineralocorticoid deficiency, potassium and acid excretion are also impaired resulting in hyperkalemia and metabolic acidosis.
- There is significant water loss and symptoms of dehydration due to salt wasting within the first two week of life. In severe form of CAH, vomiting, severe dehydration, circulatory collapse and shockdevelops in the second or third week of life.
Androgen pathway
- In the androgen synthesis pathway, 21-hydroxylase enzyme does not have a direct role; therefore with extra amount of other products from blocked cortisol and aldosterone synthesis, androgen pathway have extra precursor metabolites resulting in androgen excess in the form of dehydroepiandrosterone and androstenedione accumulation.
- On the other hand, lack of cortisol removes the negative feedback on the pituitary gland, resulting in an increase in ACTH level and consequently more increase in androgen synthesis pathway. High androgen level in 21-hydroxylase deficient women during pregnancy causes ambiguous genitalia in female fetus; also in milder forms induces hirsutism and virilization in women. Adrenal androgens produce little effect on the genitalia of male infants with severe CAH. Excess androgen can cause precocious puberty in male child.
Below is the hormonal pathway of adrenal steroids and related enzymes, also the mechanism of 21 hydroxylase deficiency symptoms.[1][2]
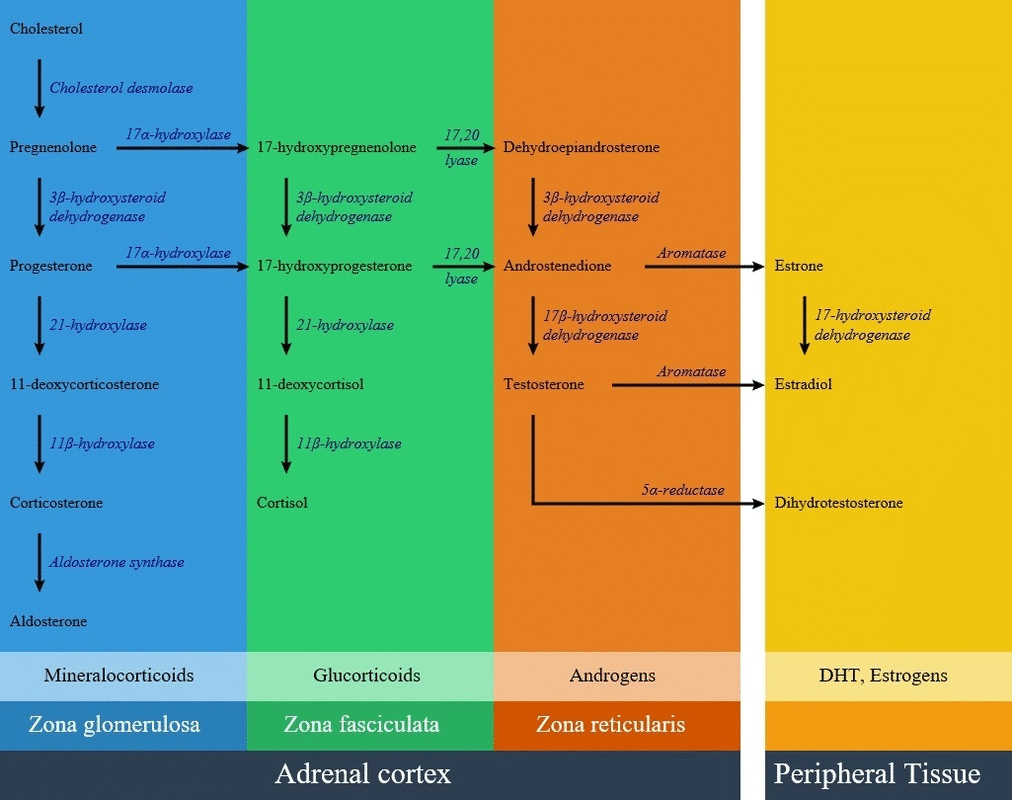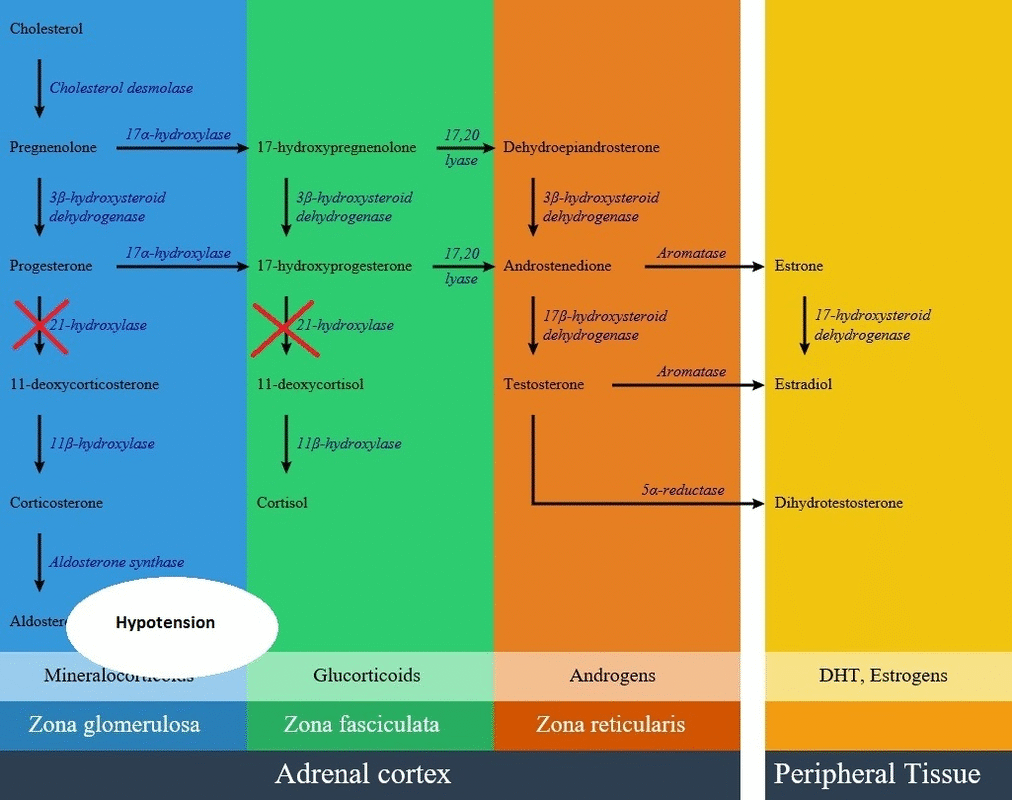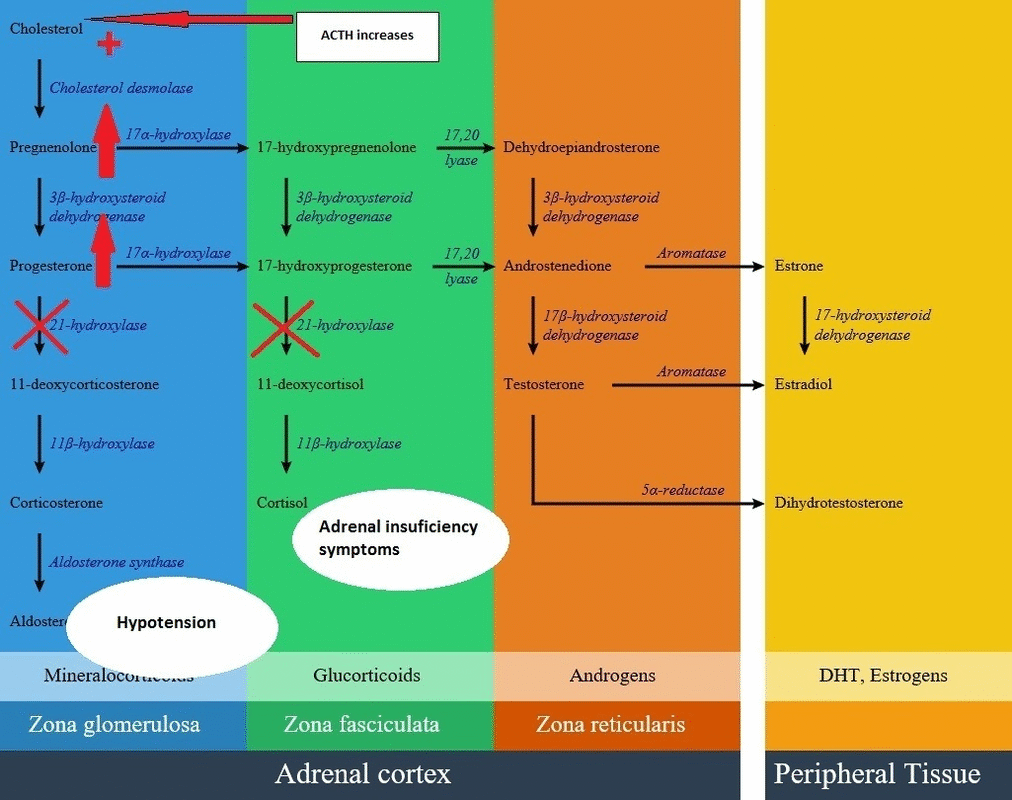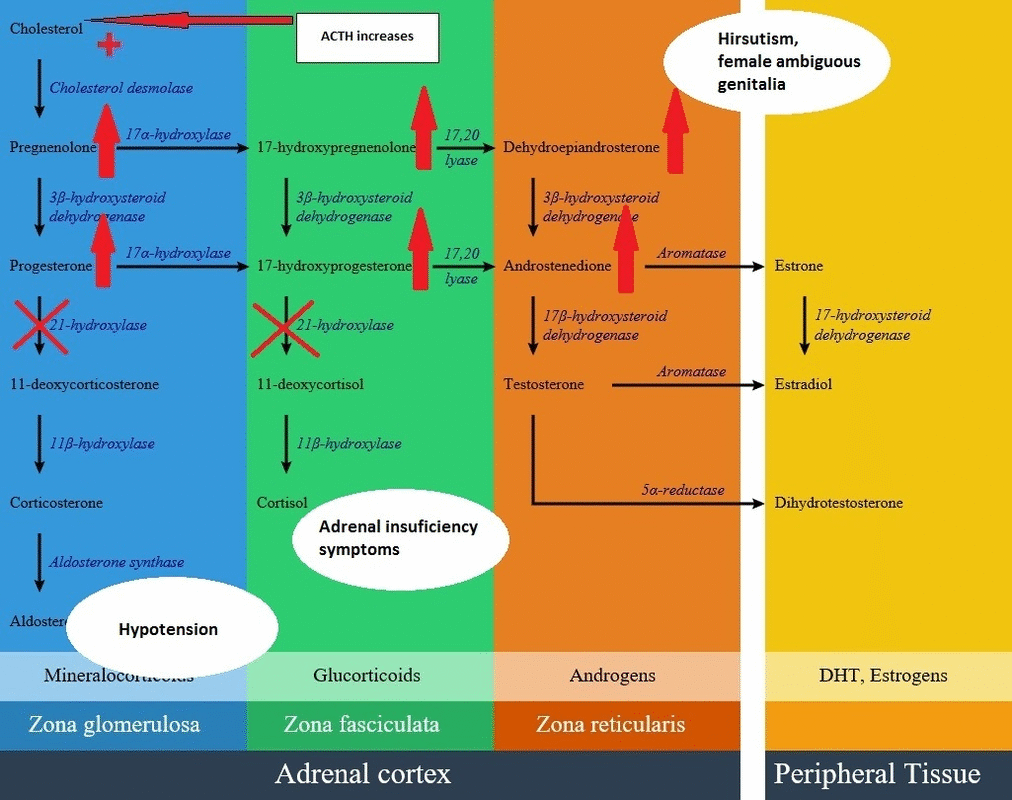
Genetics
- Congenital adrenal hyperplasia subtypes are all autosomal recessive and monogenetic. The disease manifestation follows the allele that results in a more functional enzyme, and generally correlation between genotype and phenotype is good.[4][5]
CYP21A gene
- The gene responsible for 21-hydroxylase deficiency is CYP21A. This gene is located within the human leucocyte antigen class III region of chromosome 6.
CYP21A gene has two types:
CYP21A2
- An active gene called CYP21A2, which encodes 21-hydroxylase, a cytochrome P450 type II enzyme containing 495 amino acids.
CYP21A1
- This gene is a non-functional pseudogene named CYP21A1 or CYP21P. This pseudogene produces an enzyme with no activity because it lacks eight bases from codons 110-112, which results in a stop codon.[6]
Mutation mechanisms:
- Meiotic recombination events occurs in this genomic region as a result of the high degree of sequence homology between CYP21A2 and its pseudogene CYP21A1.
- Approximately 70% of disease associated with CYP21A2 is due to gene conversion and microdeletions in CYP21A1 gene.
- Approximately 25% to 30% are chimeric genes due to large deletions.
- Approximately 1% to 2% of cases are due to de novo mutations because of high variability of the CYP21A2 locus.
- Chromosome 6 uniparental disomy is rare cause of 21-hydroxylase deficiency with an unknown prevalence.
- Gene mutations that completely inactivates CYP21A2 gene will result in the classic type and salt-wasting subtype.
- Gene mutations that maintain 1–2% of 21-hydroxylase activity will result in classic type and non-salt-wasting subtype. These patients have minimal aldosterone production that prevents a neonatal adrenal crisis.[7]
Gross Pathology
Gross pathology findings in patients with 21 hydroxylase deficiency are:[8][9]
- Enlarged adrenal glands
- Wrinkled surface of adrenal glands
- Cerebriform pattern in adrenal glands (pathognomonic sign)
- Normal ultrasound appearance
Microscopic Pathology
In 21-hydroxylase deficiency microscopic findings may include:
- Diffuse cortical hyperplasia with smaller cells
- The cell cytoplasm can be vacuolated, and often more basophilic.
- Rare mitotic figures may be present
- The hyperplastic cells typically lack features of cellular atypia.[10]
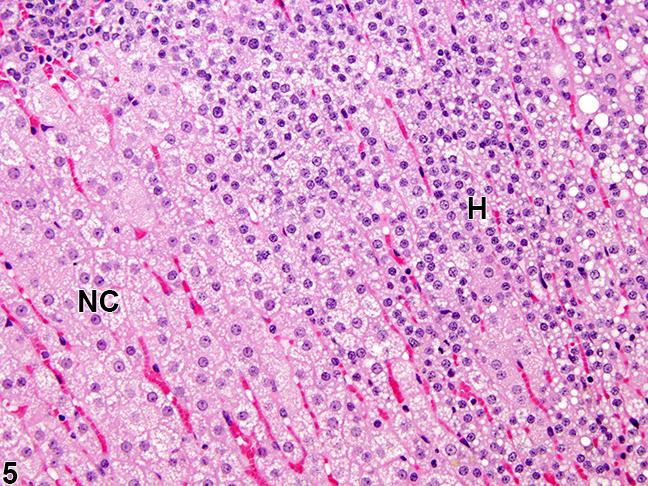 |
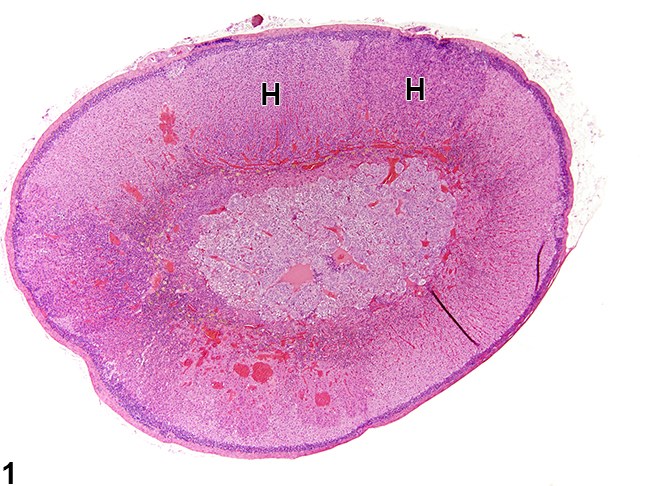 |
References
- ↑ White PC, Speiser PW (2000). "Congenital adrenal hyperplasia due to 21-hydroxylase deficiency". Endocr. Rev. 21 (3): 245–91. doi:10.1210/edrv.21.3.0398. PMID 10857554.
- ↑ Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC (2010). "Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline". J. Clin. Endocrinol. Metab. 95 (9): 4133–60. doi:10.1210/jc.2009-2631. PMC 2936060. PMID 20823466.
- ↑ "File:Adrenal Steroids Pathways.svg - Wikimedia Commons".
- ↑ Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP (2011). "Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency". J. Clin. Endocrinol. Metab. 96 (1): E161–72. doi:10.1210/jc.2010-0319. PMC 3038490. PMID 20926536.
- ↑ New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, Sun L, Zaidi M, Wilson RC, Yuen T (2013). "Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency". Proc. Natl. Acad. Sci. U.S.A. 110 (7): 2611–6. doi:10.1073/pnas.1300057110. PMC 3574953. PMID 23359698.
- ↑ White PC, New MI, Dupont B (1986). "Structure of human steroid 21-hydroxylase genes". Proc. Natl. Acad. Sci. U.S.A. 83 (14): 5111–5. PMC 323900. PMID 3487786.
- ↑ Fiet J, Gueux B, Gourmelen M, Kuttenn F, Vexiau P, Couillin P, Pham-Huu-Trung MT, Villette JM, Raux-Demay MC, Galons H (1988). "Comparison of basal and adrenocorticotropin-stimulated plasma 21-deoxycortisol and 17-hydroxyprogesterone values as biological markers of late-onset adrenal hyperplasia". J. Clin. Endocrinol. Metab. 66 (4): 659–67. doi:10.1210/jcem-66-4-659. PMID 2831244.
- ↑ Congenital adrenal hyperplasia. Dr Henry Knipe and Dr M Venkatesh . Radiopaedia.org 2015.http://radiopaedia.org/articles/congenital-adrenal-hyperplasia
- ↑ Teixeira SR, Elias PC, Andrade MT, Melo AF, Elias Junior J (2014). "The role of imaging in congenital adrenal hyperplasia". Arq Bras Endocrinol Metabol. 58 (7): 701–8. PMID 25372578.
- ↑ 10.0 10.1 10.2 "Adrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas".