Dabrafenib mesylate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dabrafenib mesylate is a BRAF Inhibitor that is FDA approved for the treatment of malignant melanoma. Common adverse reactions include peripheral edema, alopecia, hyperkeratosis, abdominal pain, constipation, anemia, arthralgia, cough.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
BRAF V600E Mutation-Positive Unresectable or Metastatic Melanoma
TAFINLAR® as a single agent is indicated for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation as detected by an FDA-approved test.
BRAF V600E or V600K Mutation-Positive Unresectable or Metastatic Melanoma
TAFINLAR, in combination with trametinib, is indicated for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations, as detected by an FDA-approved test. This indication is based on the demonstration of durable response rate [see Clinical Studies (14.2)]. Improvement in disease-related symptoms or overall survival has not been demonstrated for TAFINLAR in combination with trametinib.
Limitation of Use
TAFINLAR is not indicated for treatment of patients with wild-type BRAF melanoma.
Dosing
Recommended Dosing
- The recommended dosage regimens of TAFINLAR are:
- 150 mg orally taken twice daily, approximately 12 hours apart, as a single agent
- 150 mg orally taken twice daily, approximately 12 hours apart, in combination with trametinib 2 mg orally taken once daily
- Continue treatment until disease progression or unacceptable toxicity occurs. Take TAFINLAR as a single agent, or TAFINLAR in combination with trametinib, at least 1 hour before or 2 hours after a meal[see Clinical Pharmacology (12.3)]. Do not take a missed dose of TAFINLAR within 6 hours of the next dose of TAFINLAR. Do not open, crush, or break TAFINLAR capsule.
- When administered in combination with trametinib, take the once-daily dose of trametinib at the same time each day with either the morning dose or the evening dose of TAFINLAR.
Dose Modifications
- For New Primary Cutaneous Malignancies: No dose modifications are required.
- For New Primary Non-Cutaneous Malignancies: Permanently discontinue TAFINLAR in patients who develop RAS mutation-positive non-cutaneous malignancies. If used in combination with trametinib, no dose modifications are required for trametinib in patients who develop non-cutaneous malignancies.
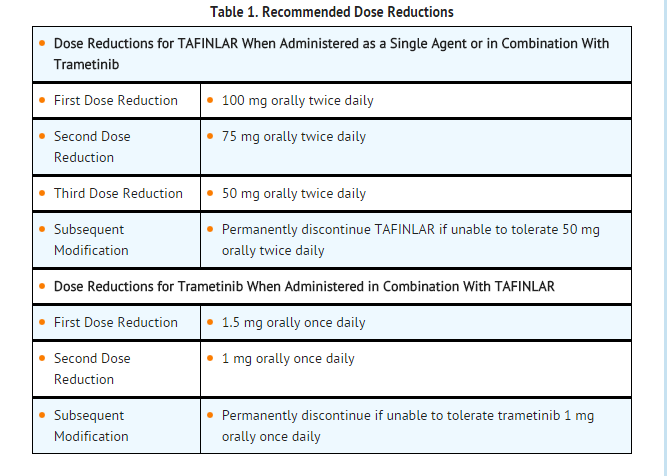

DOSAGE FORMS AND STRENGTHS
- 50 mg Capsules: Dark red capsule imprinted with ‘GS TEW’ and ‘50 mg’.
- 75 mg Capsules: Dark pink capsule imprinted with ‘GS LHF’ and ‘75 mg’.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dabrafenib mesylate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dabrafenib mesylate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Dabrafenib mesylate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dabrafenib mesylate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dabrafenib mesylate in pediatric patients.
Contraindications
- None
Warnings
- Review the Full Prescribing Information for trametinib prior to initiation of TAFINLAR in combination with trametinib. The following serious adverse reactions of trametinib as a single agent, which may occur when TAFINLAR is used in combination with trametinib, are not described in the Full Prescribing Information for TAFINLAR:
- Retinal vein occlusion
- Interstitial lung disease
New Primary Malignancies
- New primary malignancies, cutaneous and non-cutaneous, can occur when TAFINLAR is administered as a single agent or when used in combination with trametinib.
Cutaneous Malignancies:
- TAFINLAR results in an increased incidence of cutaneous squamous cell carcinoma, keratoacanthoma, and melanoma. TAFINLAR when used in combination with trametinib results in an increased incidence of basal cell carcinoma.
- In Trial 1, cutaneous squamous cell carcinomas and keratoacanthomas (cuSCC) occurred in 7% (14/187) of patients treated with TAFINLAR and in none of the patients treated with dacarbazine.
- Across clinical trials of TAFINLAR (N = 586), the incidence of cuSCC was 11%. The median time to first cuSCC was 9 weeks (range: 1 to 53 weeks). Of those patients who developed new cuSCC, approximately 33% developed one or more cuSCC with continued administration of TAFINLAR. The median time between diagnosis of the first cuSCC and the second cuSCC was 6 weeks.
- In Trial 1, the incidence of new primary malignant melanomas was 2% (3/187) for patients receiving TAFINLAR while no dacarbazine-treated patient was diagnosed with new primary malignant melanoma.
- In Trial 2, the incidence of basal cell carcinoma was increased in patients receiving TAFINLAR in combination with trametinib: 9% (5/55) of patients receiving TAFINLAR in combination with trametinib compared with 2% (1/53) of patients receiving TAFINLAR as a single agent. The range of time to diagnosis of basal cell carcinoma was 28 to 249 days in patients receiving TAFINLAR in combination with trametinib and was 197 days for the patient receiving TAFINLAR as a single agent.
- Cutaneous squamous cell carcinoma (SCC), including keratoacanthoma, occurred in 7% of patients receiving TAFINLAR in combination with trametinib and 19% of patients receiving TAFINLAR as a single agent. The range of time to diagnosis of cuSCC was 136 to197 days in the combination arm and was 9 to 197 days in the arm receiving TAFINLAR as a single agent.
- New primary melanoma occurred in 2% (1/53) of patients receiving TAFINLAR as a single agent and in none of the 55 patients receiving TAFINLAR in combination with trametinib.
- Perform dermatologic evaluations prior to initiation of TAFINLAR as a single agent or in combination with trametinib, every 2 months while on therapy, and for up to 6 months following discontinuation of TAFINLAR. No dose modifications of TAFINLAR or trametinib are required in patients who develop new primary cutaneous malignancies.
Non-cutaneous Malignancies:
- Based on its mechanism of action, TAFINLAR may promote the growth and development of malignancies with activation of RAS through mutation or other mechanisms [see Warnings and Precautions (5.2)]. In patients receiving TAFINLAR in combination with trametinib four cases of non-cutaneous malignancies were identified: KRAS mutation-positive pancreatic adenocarcinoma (n = 1), recurrent NRAS mutation-positive colorectal carcinoma (n = 1), head and neck carcinoma (n = 1), and glioblastoma (n = 1). Monitor patients receiving the combination closely for signs or symptoms of non-cutaneous malignancies. Permanently discontinue TAFINLAR for RAS mutation-positive non-cutaneous malignancies. If used in combination with trametinib, no dose modification of trametinib is required for patients who develop non-cutaneous malignancies.
Tumor Promotion in BRAF Wild-Type Melanoma
- In vitro experiments have demonstrated paradoxical activation of MAP-kinase signaling and increased cell proliferation in BRAF wild-type cells which are exposed to BRAF inhibitors. Confirm evidence of BRAF V600E or V600K mutation status prior to initiation of TAFINLAR as a single agent or combination therapy.
Hemorrhage
- Hemorrhages, including major hemorrhages defined as symptomatic bleeding in a critical area or organ, can occur when TAFINLAR is used in combination with trametinib.
- In Trial 2, treatment with TAFINLAR in combination with trametinib resulted in an increased incidence and severity of any hemorrhagic event: 16% (9/55) of patients treated with TAFINLAR in combination with trametinib compared with 2% (1/53) of patients treated with TAFINLAR as a single agent. The major hemorrhagic events of intracranial or gastric hemorrhage occurred in 5% (3/55) of patients treated with TAFINLAR in combination with trametinib compared with none of the 53 patients treated with TAFINLAR as a single agent. Intracranial hemorrhage was fatal in 4% (2/55) of patients receiving TAFINLAR in combination with trametinib.
- Permanently discontinue TAFINLAR and trametinib for all Grade 4 hemorrhagic events and for any Grade 3 hemorrhagic events that do not improve. Withhold TAFINLAR for Grade 3 hemorrhagic events; if improved resume at a lower dose level. Withhold trametinib for up to 3 weeks for Grade 3 hemorrhagic events; if improved, resume at a lower dose level.
Venous Thromboembolism
- Venous thromboembolism can occur when TAFINLAR is used in combination with trametinib.
- In Trial 2, treatment with TAFINLAR in combination with trametinib resulted in an increased incidence of deep venous thrombosis (DVT) and pulmonary embolism (PE): 7% (4/55) of patients treated with TAFINLAR in combination with trametinib compared with none of the 53 patients treated with TAFINLAR as a single agent. Pulmonary embolism was fatal in 2% (1/55) of patients receiving TAFINLAR in combination with trametinib.
- Advise patients to immediately seek medical care if they develop symptoms of DVT or PE, such as shortness of breath, chest pain, or arm or leg swelling. Permanently discontinue TAFINLAR and trametinib for life-threatening PE. Withhold trametinib and continue TAFINLAR at the same dose for uncomplicated DVT or PE; if improved within 3 weeks, trametinib may be resumed at a lower dose level.
Cardiomyopathy
- Cardiomyopathy can occur when TAFINLAR is used in combination with trametinib and with trametinib as a single agent [refer to Full Prescribing Information for trametinib].
- In Trial 2, cardiomyopathy occurred in 9% (5/55) of patients treated with TAFINLAR in combination with trametinib and in none of patients treated with TAFINLAR as a single agent. The median time to onset of cardiomyopathy in patients treated with TAFINLAR in combination with trametinib was 86 days (range: 27 to 253 days). Cardiomyopathy was identified within the first month of treatment with TAFINLAR in combination with trametinib in two of five patients. Development of cardiomyopathy resolved in all five patients following dose reduction (4/55) and/or dose interruption (1/55).
- Across clinical trials of TAFINLAR administered in combination with trametinib (N = 202), 8% of patients developed evidence of cardiomyopathy (decrease in LVEF below institutional lower limits of normal with an absolute decrease in LVEF ≥10% below baseline). Two percent demonstrated a decrease in LVEF below institutional lower limits of normal with an absolute decrease in LVEF of ≥20% below baseline.
- Assess LVEF by echocardiogram or multigated acquisition (MUGA) scan before initiation of TAFINLAR in combination with trametinib, one month after initiation, and then at 2- to 3-month intervals while on treatment with the combination. Withhold treatment with trametinib and continue TAFINLAR at the same dose if absolute LVEF value decreases by 10% from pretreatment values and is less than the lower limit of normal. For symptomatic cardiomyopathy or persistent, asymptomatic LV dysfunction that does not resolve within 4 weeks, permanently discontinue trametinib and withhold TAFINLAR. Resume TAFINLAR at the same dose level upon recovery of cardiac function.
Ocular Toxicities
Retinal Pigment Epithelial Detachment (RPED):
- Retinal pigment epithelial detachments (RPED) can occur when TAFINLAR is used in combination with trametinib and with trametinib as a single agent [refer to Full Prescribing Information for trametinib]. Retinal detachments resulting from trametinib are often bilateral and multifocal, occurring in the macular region of the retina.
- In Trial 2, ophthalmologic examinations including retinal evaluation were performed pretreatment and at regular intervals during treatment. RPED occurred in 2% (1/55) of patients receiving TAFINLAR in combination with trametinib. Across clinical trials of TAFINLAR administered in combination with trametinib (N = 202), the incidence of RPED was 1% (2/202).
- Perform ophthalmological evaluation at any time a patient reports visual disturbances and compare with baseline, if available. If TAFINLAR is used in combination with trametinib, do not modify the dose of TAFINLAR. Withhold trametinib if RPED is diagnosed. If resolution of the RPED is documented on repeat ophthalmological evaluation within 3 weeks, resume trametinib at a lower dose level. Discontinue trametinib if no improvement after 3 weeks.
Uveitis and Iritis:
- Uveitis and iritis can occur when TAFINLAR is administered as a single agent or when used in combination with trametinib.
- Uveitis (including iritis) occurred in 1% (6/586) of patients treated with TAFINLAR as a single agent and uveitis occurred in 1% (2/202) of patients treated with TAFINLAR in combination with trametinib. Symptomatic treatment employed in clinical trials included steroid and mydriatic ophthalmic drops. Monitor patients for visual signs and symptoms of uveitis (e.g., change in vision, photophobia, eye pain). If diagnosed, withhold TAFINLAR for up to 6 weeks until uveitis/iritis resolves to Grade 0-1. If TAFINLAR is used in combination with trametinib, do not modify the dose of trametinib.
Serious Febrile Reactions
- Serious febrile reactions and fever of any severity complicated by hypotension, rigors or chills, dehydration, or renal failure, can occur when TAFINLAR is administered as a single agent or when used in combination with trametinib. The incidence and severity of pyrexia are increased when TAFINLAR is used in combination with trametinib compared with TAFINLAR as a single agent.
- In Trial 1, the incidence of fever (serious and non-serious) was 28% in patients treated with TAFINLAR and 10% in patients treated with dacarbazine. In patients treated with TAFINLAR, the median time to initial onset of fever (any severity) was 11 days (range: 1 to 202 days) and the median duration of fever was 3 days (range: 1 to 129 days). Serious febrile reactions and fever of any severity complicated by hypotension, rigors or chills occurred in 3.7% (7/187) of patients treated with TAFINLAR and in none of the 59 patients treated with dacarbazine.
- In Trial 2, the incidence of fever (serious and non-serious) was 71% (39/55) in patients treated with TAFINLAR in combination with trametinib and 26% (14/53) in patients treated with TAFINLAR as a single agent. Serious febrile reactions and fever of any severity complicated by hypotension, rigors or chills occurred in 25% (14/55) of patients treated with TAFINLAR in combination with trametinib compared with 2% (1/53) of patients treated with TAFINLAR as a single agent. Fever was complicated with chills/rigors in 51% (28/55), dehydration in 9% (5/55), renal failure in 4% (2/55), and syncope in 4% (2/55) of patients in Trial 2.
- In patients treated with TAFINLAR in combination with trametinib, the median time to initial onset of fever was 30 days compared with 19 days in patients treated with TAFINLAR as a single agent; the median duration of fever was 6 days with the combination compared with 4 days with TAFINLAR as a single agent.
- Across clinical trials of TAFINLAR administered in combination with trametinib (N = 202), the incidence of pyrexia was 57% (116/202).
- Withhold TAFINLAR for fever of 101.3ºF or higher. Withhold trametinib for any fever higher than 104ºF. Withhold TAFINLAR, and trametinib if used in combination, for any serious febrile reaction or fever complicated by hypotension, rigors or chills, dehydration, or renal failure and evaluate for signs and symptoms of infection. Refer to Table 2 for recommended dose modifications for adverse reactions [see Dosage and Administration (2.3)]. Prophylaxis with antipyretics may be required when resuming TAFINLAR or trametinib.
Serious Skin Toxicity
- Serious skin toxicity can occur when TAFINLAR is used in combination with trametinib and with trametinib as a single agent [refer to Full Prescribing Information for trametinib].
- In Trial 2, the incidence of any skin toxicity was similar for patients receiving TAFINLAR in combination with trametinib (65% [36/55]) compared with patients receiving TAFINLAR as a single agent (68% [36/53]). The median time to onset of skin toxicity in patients treated with TAFINLAR in combination with trametinib was 37 days (range: 1 to 225 days) and median time to resolution of skin toxicity was 33 days (range: 3 to 421 days). No patient required dose reduction or permanent discontinuation of TAFINLAR or trametinib for skin toxicity.
- Across clinical trials of TAFINLAR in combination with trametinib (N = 202), severe skin toxicity and secondary infections of the skin requiring hospitalization occurred in 2.5% (5/202) of patients treated with TAFINLAR in combination with trametinib.
- Withhold TAFINLAR, and trametinib if used in combination, for intolerable or severe skin toxicity. TAFINLAR and trametinib may be resumed at lower dose levels in patients with improvement or recovery from skin toxicity within 3 weeks [see Dosage and Administration (2.3)].
Hyperglycemia
- Hyperglycemia can occur when TAFINLAR is administered as a single agent or when used in combination with trametinib.
- In Trial 1, 5 of 12 patients with a history of diabetes required more intensive hypoglycemic therapy while taking TAFINLAR. The incidence of Grade 3 hyperglycemia based on laboratory values was 6% (12/187) in patients treated with TAFINLAR compared with none of the dacarbazine-treated patients.
- In Trial 2, the incidence of Grade 3 hyperglycemia based on laboratory values was 5% (3/55) in patients treated with TAFINLAR in combination with trametinib compared with 2% (1/53) in patients treated with TAFINLAR as a single agent.
- Monitor serum glucose levels as clinically appropriate when TAFINLAR is administered as a single agent or when used in combination with trametinib in patients with pre-existing diabetes or hyperglycemia. Advise patients to report symptoms of severe hyperglycemia such as excessive thirst or any increase in the volume or frequency of urination.
Glucose-6-Phosphate Dehydrogenase Deficiency
- TAFINLAR, which contains a sulfonamide moiety, confers a potential risk of hemolytic anemia in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. Closely observe patients with G6PD deficiency for signs of hemolytic anemia.
Embryofetal Toxicity
- Based on its mechanism of action, TAFINLAR can cause fetal harm when administered to a pregnant woman. Dabrafenib was teratogenic and embryotoxic in rats at doses three times greater than the human exposure at the recommended clinical dose. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
- Advise female patients of reproductive potential to use a highly effective non-hormonal method of contraception since TAFINLAR can render hormonal contraceptives ineffective, during treatment and for at least 2 weeks after treatment with TAFINLAR or for 4 months after treatment with TAFINLAR in combination with trametinib. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking TAFINLAR.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in greater detail in another section of the label:
- New Primary Malignancies
- Tumor Promotion in BRAF Wild-Type Melanoma
- Hemorrhage
- Venous Thromboembolism
- Cardiomyopathy
- Ocular Toxicities
- Serious Febrile Reactions
- Serious Skin Toxicity
- Hyperglycemia
- Glucose-6-Phosphate Dehydrogenase Deficiency
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data described in the Warnings and Precautions section and below reflect exposure to TAFINLAR as a single agent and in combination with trametinib.
BRAF V600E Unresectable or Metastatic Melanoma:
- The safety of TAFINLAR as a single agent was evaluated in 586 patients with BRAF V600 mutation-positive unresectable or metastatic melanoma, previously treated or untreated, who received TAFINLAR 150 mg orally twice daily until disease progression or unacceptable toxicity, including 181 patients treated for at least 6 months and 86 additional patients treated for more than 12 months. TAFINLAR was studied in open-label, single-arm trials and in an open-label, randomized, active-controlled trial. The median daily dose of TAFINLAR was 300 mg (range: 118 to 300 mg).
- Table 3 and Table 4 present adverse drug reactions and laboratory abnormalities identified from analyses of Trial 1 [see Clinical Studies (14.1)]. Trial 1, a multicenter, international, open-label, randomized (3:1), controlled trial allocated 250 patients with unresectable or metastatic BRAF V600E mutation-positive melanoma to receive TAFINLAR 150 mg orally twice daily (n = 187) or dacarbazine 1,000 mg/m2 intravenously every 3 weeks (n = 63). The trial excluded patients with abnormal left ventricular ejection fraction or cardiac valve morphology (≥Grade 2), corrected QT interval ≥480 milliseconds on electrocardiogram, or a known history of glucose-6-phosphate dehydrogenase deficiency. The median duration on treatment was 4.9 months for patients treated with TAFINLAR and 2.8 months for dacarbazine-treated patients. The population exposed to TAFINLAR was 60% male, 99% white, and had a median age of 53 years.
- The most commonly occurring adverse reactions (≥20%) in patients treated with TAFINLAR were, in order of decreasing frequency: hyperkeratosis, headache, pyrexia, arthralgia, papilloma, alopecia, and palmar-plantar erythrodysesthesia syndrome (PPES).
- The incidence of adverse events resulting in permanent discontinuation of study medication in Trial 1 was 3% for patients treated with TAFINLAR and 3% for patients treated with dacarbazine. The most frequent (≥2%) adverse reactions leading to dose reduction of TAFINLAR were pyrexia (9%), PPES (3%), chills (3%), fatigue (2%), and headache (2%).
- Other clinically important adverse reactions observed in <10% of patients (N = 586) treated with TAFINLAR were:
- Gastrointestinal Disorders: Pancreatitis.
- Immune System Disorders: Hypersensitivity manifesting as bullous rash.
Renal and Urinary Disorders: Interstitial nephritis.
BRAF V600E or V600K Unresectable or Metastatic Melanoma:
The safety of TAFINLAR in combination with trametinib was evaluated in Trial 2 and other trials consisting of a total of 202 patients with BRAF V600 mutation-positive unresectable or metastatic melanoma who received TAFINLAR 150 mg orally twice daily in combination with trametinib 2 mg orally once daily until disease progression or unacceptable toxicity. Among these 202 patients, 66 (33%) were exposed to TAFINLAR and 68 (34%) were exposed to trametinib for greater than 6 to 12 months while 40 (20%) were exposed to TAFINLAR and 36 (18%) were exposed to trametinib for greater than one year. The median age was 54 years, 57% were male, and >99% were white.
Table 5 presents adverse reactions from Trial 2, a multicenter, open-label, randomized trial of 162 patients with BRAF V600E or V600K mutation-positive melanoma receiving TAFINLAR 150 mg twice daily in combination with trametinib 2 mg orally once daily (n = 55), TAFINLAR 150 mg orally twice daily in combination with trametinib 1 mg once daily (n = 54), and TAFINLAR as a single agent 150 mg orally twice daily (n = 53). Patients with abnormal LVEF, history of acute coronary syndrome within 6 months, current evidence of Class II or greater congestive heart failure (New York Heart Association), history RVO or RPED, QTc interval ≥480 msec, treatment refractory hypertension, uncontrolled arrhythmias, history of pneumonitis or interstitial lung disease, or a known history of G6PD deficiency were excluded. The median duration of treatment was 10.9 months for both TAFINLAR and trametinib (2-mg orally once-daily treatment group) when used in combination, 10.6 months for both TAFINLAR and trametinib (1-mg orally once-daily treatment group) when used in combination, and 6.1 months for TAFINLAR as a single agent.
In Trial 2, 13% of patients receiving TAFINLAR in combination with trametinib experienced adverse reactions resulting in permanent discontinuation of trial medication(s). The most common adverse reaction resulting in permanent discontinuation was pyrexia (4%). Adverse reactions led to dose reductions in 49% and dose interruptions in 67% of patients treated with TAFINLAR in combination with trametinib. Pyrexia, chills, and nausea were the most common reasons cited for dose reductions and pyrexia, chills, and decreased ejection fraction were the most common reasons cited for dose interruptions of TAFINLAR and trametinib when used in combination.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Dabrafenib mesylate in the drug label.
Drug Interactions
There is limited information regarding Dabrafenib mesylate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Dabrafenib mesylate in women who are pregnant.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dabrafenib mesylate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dabrafenib mesylate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Dabrafenib mesylate with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Dabrafenib mesylate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Dabrafenib mesylate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Dabrafenib mesylate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dabrafenib mesylate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dabrafenib mesylate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dabrafenib mesylate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dabrafenib mesylate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dabrafenib mesylate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Dabrafenib mesylate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Dabrafenib mesylate in the drug label.
Overdosage
There is limited information regarding Dabrafenib mesylate overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Dabrafenib mesylate Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Dabrafenib mesylate Mechanism of Action in the drug label.
Structure
There is limited information regarding Dabrafenib mesylate Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Dabrafenib mesylate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Dabrafenib mesylate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Dabrafenib mesylate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Dabrafenib mesylate in the drug label.
How Supplied
Storage
There is limited information regarding Dabrafenib mesylate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Dabrafenib mesylate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dabrafenib mesylate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Dabrafenib mesylate in the drug label.
Precautions with Alcohol
- Alcohol-Dabrafenib mesylate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Dabrafenib mesylate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Dabrafenib mesylate |Label Name=Dabrafenib mesylate11.png
}}
{{#subobject:
|Label Page=Dabrafenib mesylate |Label Name=Dabrafenib mesylate11.png
}}