COVID-19-associated pulmonary embolism: Difference between revisions
No edit summary |
|||
| Line 4: | Line 4: | ||
* In May 2020, various [[Autopsy|autopsies]] studies revealed [[pulmonary embolism]] to be the common cause of death in [[COVID-19]] infected patients. | * In May 2020, various [[Autopsy|autopsies]] studies revealed [[pulmonary embolism]] to be the common cause of death in [[COVID-19]] infected patients. | ||
* These patients in their mid-70s had preexisting medical conditions such as cardiac diseases, [[hypertension]], [[diabetes]], and [[obesity]]. | * These patients in their mid-70s had preexisting medical conditions such as cardiac diseases, [[hypertension]], [[diabetes]], and [[obesity]].<ref name="Wichmann Sperhake Lütgehetmann Steurer p.">{{cite journal | last=Wichmann | first=Dominic | last2=Sperhake | first2=Jan-Peter | last3=Lütgehetmann | first3=Marc | last4=Steurer | first4=Stefan | last5=Edler | first5=Carolin | last6=Heinemann | first6=Axel | last7=Heinrich | first7=Fabian | last8=Mushumba | first8=Herbert | last9=Kniep | first9=Inga | last10=Schröder | first10=Ann Sophie | last11=Burdelski | first11=Christoph | last12=de Heer | first12=Geraldine | last13=Nierhaus | first13=Axel | last14=Frings | first14=Daniel | last15=Pfefferle | first15=Susanne | last16=Becker | first16=Heinrich | last17=Bredereke-Wiedling | first17=Hanns | last18=de Weerth | first18=Andreas | last19=Paschen | first19=Hans-Richard | last20=Sheikhzadeh-Eggers | first20=Sara | last21=Stang | first21=Axel | last22=Schmiedel | first22=Stefan | last23=Bokemeyer | first23=Carsten | last24=Addo | first24=Marylyn M. | last25=Aepfelbacher | first25=Martin | last26=Püschel | first26=Klaus | last27=Kluge | first27=Stefan | title=Autopsy Findings and Venous Thromboembolism in Patients With COVID-19 | journal=Annals of Internal Medicine | publisher=American College of Physicians | date=2020-05-06 | issn=0003-4819 | doi=10.7326/m20-2003 | page=}}</ref> | ||
* These studies highlight the role of [[hypercoagulability]] as the main contributor to the fatality in these patients. | * These studies highlight the role of [[hypercoagulability]] as the main contributor to the fatality in these patients. | ||
* Various studies have described [[Virchow's triad]] to be the main component of the hypercoagulable state in these patients. | * Various studies have described [[Virchow's triad]] to be the main component of the hypercoagulable state in these patients. | ||
| Line 38: | Line 38: | ||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Description of the underlying mechanism}} | !style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Description of the underlying mechanism}} | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Endothelial cells|Endothelial cells dysfunction]] | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Endothelial cells|Endothelial cells dysfunction]] <ref name="Teuwen Geldhof Pasut Carmeliet p.">{{cite journal | last=Teuwen | first=Laure-Anne | last2=Geldhof | first2=Vincent | last3=Pasut | first3=Alessandra | last4=Carmeliet | first4=Peter | title=COVID-19: the vasculature unleashed | journal=Nature Reviews Immunology | publisher=Springer Science and Business Media LLC | date=2020-05-21 | issn=1474-1733 | doi=10.1038/s41577-020-0343-0 | page=}}</ref> | ||
| | | | ||
*It has been proposed that [[endothelial cells]] contribute towards the initiation and propagation of [[ARDS]] by changing the vascular barrier permeability, increasing the chance of procoagulative state that leads to endotheliitis and infiltration of inflammatory cells in the [[Pulmonary vasculature|pulmonary vasculature.]] | *It has been proposed that [[endothelial cells]] contribute towards the initiation and propagation of [[ARDS]] by changing the vascular barrier permeability, increasing the chance of procoagulative state that leads to endotheliitis and infiltration of inflammatory cells in the [[Pulmonary vasculature|pulmonary vasculature.]] | ||
| Line 48: | Line 48: | ||
|Most hospitalized critically ill immobile [[COVID-19]] patients are prone to stasis of blood flow leading to another contributor towards the [[pathogenesis]] of [[pulmonary embolism]]. | |Most hospitalized critically ill immobile [[COVID-19]] patients are prone to stasis of blood flow leading to another contributor towards the [[pathogenesis]] of [[pulmonary embolism]]. | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Hypercoagulable state]] | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Hypercoagulable state]] <ref name="Panigada Bottino Tagliabue Grasselli p.">{{cite journal | last=Panigada | first=Mauro | last2=Bottino | first2=Nicola | last3=Tagliabue | first3=Paola | last4=Grasselli | first4=Giacomo | last5=Novembrino | first5=Cristina | last6=Chantarangkul | first6=Veena | last7=Pesenti | first7=Antonio | last8=Peyvandi | first8=Fora | last9=Tripodi | first9=Armando | title=Hypercoagulability of COVID‐19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis | journal=Journal of Thrombosis and Haemostasis | publisher=Wiley | date=2020-04-17 | issn=1538-7933 | doi=10.1111/jth.14850 | page=}}</ref> | ||
| | | | ||
Various clinical studies have reported different [[prothrombotic factors]] in patients who are critically ill and are hospitalized due to [[COVID-19|COVID-19.]] These studies report various key lab factors that play an important role in the pathogenesis of pulmonary embolism. | Various clinical studies have reported different [[prothrombotic factors]] in patients who are critically ill and are hospitalized due to [[COVID-19|COVID-19.]] These studies report various key lab factors that play an important role in the pathogenesis of pulmonary embolism. | ||
| Line 105: | Line 105: | ||
*Various case reports and case series report relatively high incidence of [[pulmonary embolism]] in ICU patients. | *Various case reports and case series report relatively high incidence of [[pulmonary embolism]] in ICU patients. | ||
* The incidence of thrombotic complications is reported to be 31 % in one study. In this study pulmonary embolism was the most common [[Thrombosis|thrombotic]] complication. | * The incidence of thrombotic complications is reported to be 31 % in one study. In this study pulmonary embolism was the most common [[Thrombosis|thrombotic]] complication..<ref name="Klok Kruip van der Meer Arbous 2020 pp. 145–147">{{cite journal | last=Klok | first=F.A. | last2=Kruip | first2=M.J.H.A. | last3=van der Meer | first3=N.J.M. | last4=Arbous | first4=M.S. | last5=Gommers | first5=D.A.M.P.J. | last6=Kant | first6=K.M. | last7=Kaptein | first7=F.H.J. | last8=van Paassen | first8=J. | last9=Stals | first9=M.A.M. | last10=Huisman | first10=M.V. | last11=Endeman | first11=H. | title=Incidence of thrombotic complications in critically ill ICU patients with COVID-19 | journal=Thrombosis Research | publisher=Elsevier BV | volume=191 | year=2020 | issn=0049-3848 | doi=10.1016/j.thromres.2020.04.013 | pages=145–147}}</ref> | ||
*Another study reported an overall 24% [[cumulative incidence]] of pulmonary embolism in patients with COVID-19 pneumonia, 50% (30–70%) in ICU and 18% (12–27%) in other patients. | *Another study reported an overall 24% [[cumulative incidence]] of pulmonary embolism in patients with COVID-19 pneumonia, 50% (30–70%) in ICU and 18% (12–27%) in other patients.<ref name="Bompard Monnier Saab Tordjman p=2001365">{{cite journal | last=Bompard | first=Florian | last2=Monnier | first2=Hippolyte | last3=Saab | first3=Ines | last4=Tordjman | first4=Mickael | last5=Abdoul | first5=Hendy | last6=Fournier | first6=Laure | last7=Sanchez | first7=Olivier | last8=Lorut | first8=Christine | last9=Chassagnon | first9=Guillaume | last10=Revel | first10=Marie-pierre | title=Pulmonary embolism in patients with Covid-19 pneumonia | journal=European Respiratory Journal | publisher=European Respiratory Society (ERS) | date=2020-05-12 | issn=0903-1936 | doi=10.1183/13993003.01365-2020 | page=2001365}}</ref> | ||
* In Non-ICU settings (In-patient), [[pulmonary embolism]] is reported to occur in 3% percent of patients in one study. | * In Non-ICU settings (In-patient), [[pulmonary embolism]] is reported to occur in 3% percent of patients in one study.<ref name="Middeldorp Coppens van Haaps Foppen p.">{{cite journal | last=Middeldorp | first=Saskia | last2=Coppens | first2=Michiel | last3=van Haaps | first3=Thijs F. | last4=Foppen | first4=Merijn | last5=Vlaar | first5=Alexander P. | last6=Müller | first6=Marcella C.A. | last7=Bouman | first7=Catherine C.S. | last8=Beenen | first8=Ludo F.M. | last9=Kootte | first9=Ruud S. | last10=Heijmans | first10=Jarom | last11=Smits | first11=Loek P. | last12=Bonta | first12=Peter I. | last13=van Es | first13=Nick | title=Incidence of venous thromboembolism in hospitalized patients with COVID‐19 | journal=Journal of Thrombosis and Haemostasis | publisher=Wiley | date=2020-05-05 | issn=1538-7933 | doi=10.1111/jth.14888 | page=}}</ref> | ||
==Risk Factors== | ==Risk Factors== | ||
Multivariate analysis showed following risk factors that predispose a patient of COVID-19 to [[pulmonary embolism]] | Multivariate analysis showed following risk factors that predispose a patient of COVID-19 to [[pulmonary embolism]]. <ref name="Poyiadi Cormier Patel Hadied p=201955">{{cite journal | last=Poyiadi | first=Neo | last2=Cormier | first2=Peter | last3=Patel | first3=Parth Y. | last4=Hadied | first4=Mohamad O. | last5=Bhargava | first5=Pallavi | last6=Khanna | first6=Kanika | last7=Nadig | first7=Jeffrey | last8=Keimig | first8=Thomas | last9=Spizarny | first9=David | last10=Reeser | first10=Nicholas | last11=Klochko | first11=Chad | last12=Peterson | first12=Edward L. | last13=Song | first13=Thomas | title=Acute Pulmonary Embolism and COVID-19 | journal=Radiology | publisher=Radiological Society of North America (RSNA) | date=2020-05-14 | issn=0033-8419 | doi=10.1148/radiol.2020201955 | page=201955}}</ref> | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 150: | Line 150: | ||
*[[Pulmonary embolism]] can present with no symptoms to [[shock]] and even [[Sudden cardiac death|sudden cardiac arrest]]. | *[[Pulmonary embolism]] can present with no symptoms to [[shock]] and even [[Sudden cardiac death|sudden cardiac arrest]]. | ||
* The most common symptoms that were observed in Prospective Investigation of Pulmonary Embolism Diagnosis II (PIOPED II) trial include: | * The most common symptoms that were observed in Prospective Investigation of Pulmonary Embolism Diagnosis II (PIOPED II) trial include:<ref name="Stein Beemath Matta Weg 2007 pp. 871–879">{{cite journal | last=Stein | first=Paul D. | last2=Beemath | first2=Afzal | last3=Matta | first3=Fadi | last4=Weg | first4=John G. | last5=Yusen | first5=Roger D. | last6=Hales | first6=Charles A. | last7=Hull | first7=Russell D. | last8=Leeper | first8=Kenneth V. | last9=Sostman | first9=H. Dirk | last10=Tapson | first10=Victor F. | last11=Buckley | first11=John D. | last12=Gottschalk | first12=Alexander | last13=Goodman | first13=Lawrence R. | last14=Wakefied | first14=Thomas W. | last15=Woodard | first15=Pamela K. | title=Clinical Characteristics of Patients with Acute Pulmonary Embolism: Data from PIOPED II | journal=The American Journal of Medicine | publisher=Elsevier BV | volume=120 | issue=10 | year=2007 | issn=0002-9343 | doi=10.1016/j.amjmed.2007.03.024 | pages=871–879}}</ref> | ||
**[[Dyspnea]] that is sudden in onset at rest or exertion (73%) | **[[Dyspnea]] that is sudden in onset at rest or exertion (73%) | ||
**[[Pleuritic pain]] (44%) | **[[Pleuritic pain]] (44%) | ||
| Line 158: | Line 158: | ||
==== Physical Examination ==== | ==== Physical Examination ==== | ||
On physical examination following signs can be demonstrated in COVID-19 patients. | On physical examination following signs can be demonstrated in COVID-19 patients.<ref name="Stein Beemath Matta Weg 2007 pp. 871–879">{{cite journal | last=Stein | first=Paul D. | last2=Beemath | first2=Afzal | last3=Matta | first3=Fadi | last4=Weg | first4=John G. | last5=Yusen | first5=Roger D. | last6=Hales | first6=Charles A. | last7=Hull | first7=Russell D. | last8=Leeper | first8=Kenneth V. | last9=Sostman | first9=H. Dirk | last10=Tapson | first10=Victor F. | last11=Buckley | first11=John D. | last12=Gottschalk | first12=Alexander | last13=Goodman | first13=Lawrence R. | last14=Wakefied | first14=Thomas W. | last15=Woodard | first15=Pamela K. | title=Clinical Characteristics of Patients with Acute Pulmonary Embolism: Data from PIOPED II | journal=The American Journal of Medicine | publisher=Elsevier BV | volume=120 | issue=10 | year=2007 | issn=0002-9343 | doi=10.1016/j.amjmed.2007.03.024 | pages=871–879}}</ref> | ||
====== General ====== | ====== General ====== | ||
| Line 187: | Line 187: | ||
=====Laboratory findings===== | =====Laboratory findings===== | ||
Lab findings of different case studies of patients having pulmonary embolism due to COVID-19 are given as | Lab findings of different case studies of patients having pulmonary embolism due to COVID-19 are given as <ref name="Bikdeli Madhavan Jimenez Chuich 2020 pp. 2950–29732">{{cite journal | last=Bikdeli | first=Behnood | last2=Madhavan | first2=Mahesh V. | last3=Jimenez | first3=David | last4=Chuich | first4=Taylor | last5=Dreyfus | first5=Isaac | last6=Driggin | first6=Elissa | last7=Nigoghossian | first7=Caroline Der | last8=Ageno | first8=Walter | last9=Madjid | first9=Mohammad | last10=Guo | first10=Yutao | last11=Tang | first11=Liang V. | last12=Hu | first12=Yu | last13=Giri | first13=Jay | last14=Cushman | first14=Mary | last15=Quéré | first15=Isabelle | last16=Dimakakos | first16=Evangelos P. | last17=Gibson | first17=C. Michael | last18=Lippi | first18=Giuseppe | last19=Favaloro | first19=Emmanuel J. | last20=Fareed | first20=Jawed | last21=Caprini | first21=Joseph A. | last22=Tafur | first22=Alfonso J. | last23=Burton | first23=John R. | last24=Francese | first24=Dominic P. | last25=Wang | first25=Elizabeth Y. | last26=Falanga | first26=Anna | last27=McLintock | first27=Claire | last28=Hunt | first28=Beverley J. | last29=Spyropoulos | first29=Alex C. | last30=Barnes | first30=Geoffrey D. | last31=Eikelboom | first31=John W. | last32=Weinberg | first32=Ido | last33=Schulman | first33=Sam | last34=Carrier | first34=Marc | last35=Piazza | first35=Gregory | last36=Beckman | first36=Joshua A. | last37=Steg | first37=P. Gabriel | last38=Stone | first38=Gregg W. | last39=Rosenkranz | first39=Stephan | last40=Goldhaber | first40=Samuel Z. | last41=Parikh | first41=Sahil A. | last42=Monreal | first42=Manuel | last43=Krumholz | first43=Harlan M. | last44=Konstantinides | first44=Stavros V. | last45=Weitz | first45=Jeffrey I. | last46=Lip | first46=Gregory Y.H. | title=COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up | journal=Journal of the American College of Cardiology | publisher=Elsevier BV | volume=75 | issue=23 | year=2020 | issn=0735-1097 | doi=10.1016/j.jacc.2020.04.031 | pages=2950–2973}}</ref> | ||
*Elevated [[D-dimer|d-dimers]] | *Elevated [[D-dimer|d-dimers]] | ||
Revision as of 15:27, 29 June 2020
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Usman Ali Akbar, M.B.B.S.[2] Sara Mohsin, M.D.[3]
Overview
- In May 2020, various autopsies studies revealed pulmonary embolism to be the common cause of death in COVID-19 infected patients.
- These patients in their mid-70s had preexisting medical conditions such as cardiac diseases, hypertension, diabetes, and obesity.[1]
- These studies highlight the role of hypercoagulability as the main contributor to the fatality in these patients.
- Various studies have described Virchow's triad to be the main component of the hypercoagulable state in these patients.
Historical Perspective
- In late 2019, a novel coronavirus had been identified in Wuhan ,China which has now reached a pandemic state across whole world.
- Various case reports and case series have suggested hypercoagulability to be one of the cause of death in COVID-19 patients.
- Proposed mechanism of multiple organ dysfunction that occurs in COVID-19 patients are multifactorial but they include a hypercoagulable state with micro and macro-circulatory thrombosis.
- Based on these case reports and case series, various guidelines have been proposed now to initiate anticoagulants in critically ill patients and those who are admitted to the hospital.
Classification
Acute Pulmonary Embolism
- Pathologically an embolus is said to be acute when it is situated centrally within in the vascular lumen, or in other case it causes the occlusion of vessel. It can cause immediate occurence of symptoms.
Chronic Pulmonary Embolism
- An embolus is said to be chronic , if it is eccentric and lies in lies with the vessel wall.
- It occouleds the lumen of vessel wall by more than 50 %.
- There is also evidence of recanalization within the thrombus.
- Chronic thromboembolism can cause pulmonary hypertension especially when there is >3 months of effective anticoagulation therapy and one of the following criteria. a) Mean pulmonary arterial pressure greater than or equal to 25 mmHg b) Pulmonary arterial wedge pressure less than or equal to 15 mmgHg c) Abnormal lung V/Q scan or imaging findings suggestive of chronic pulmonary embolism on CTPA, CMR, CPA.
Pathophysiology
- As data on COVID-19 has been incomplete and evolving, the pathogenesis of pulmonary embolism has not yet been completely understood. Various contributors to the pathogenesis of pulmonary embolism in these patients are listed in the table below:
| Pathology | Description of the underlying mechanism |
|---|---|
| Endothelial cells dysfunction [2] |
|
| Stasis | Most hospitalized critically ill immobile COVID-19 patients are prone to stasis of blood flow leading to another contributor towards the pathogenesis of pulmonary embolism. |
| Hypercoagulable state [3] |
Various clinical studies have reported different prothrombotic factors in patients who are critically ill and are hospitalized due to COVID-19. These studies report various key lab factors that play an important role in the pathogenesis of pulmonary embolism.
|

Causes
Recently, SARS-CoV-2 has been associated with pulmonary embolism and other coagulopathic disorders. Other than SARS-CoV-2, pulmonary embolism can be caused by a number of different factors
| Hereditary Causes | Comorbidities | Miscellaneous |
|---|---|---|
| Factor V Leiden Mutation | Heart failure | Surgery |
| Factor C & S deficiency | Congenital heart disease | Pregnancy |
| Antithrombin deficiency | Antiphospholipid syndrome | OCPs |
| Obesity | Immobilization | |
| Myeloproliferative Disorders | Trauma | |
| Paroxysmal nocturnal hemoglobinuria | Malignancy |
Differentiating Pulmonary Embolism from other Diseases
Pulmonary embolism in COVID-19 patients can be sudden and can mimic symptoms of other disorders like pneumonia and ARDS. Therefore it has been suggested there should be lower threshold of imaging like DVT or CTPA reserved for pulmonary embolism in COVID-19 patients admitted to the ICU setting.
- Differentiating from heart failure : Acute congestive Heart failure presents in context of previous myocardial infarction,hypertension any other previous co-morbidities. There is usually no history of associated chest pain, hemoptysis or low grade fever. Additionally there will be signs of volume overload e.g, distended jugular vein, peripheral edema, hepatomegaly,pulmonary edema etc present in congestive heart failure patient
- Differentiating from Pericarditis : Acute pericarditis presents similar to acute pulmonary embolism. However there is history of recent viral infection, underlying disease such as uremia, myocardial infarction and can also occur due to malignancy. There would be ST-segment elevation and PR depression on ECG. The chest pain will be relieved by sitting up and leaning forward.
- Differentiating from Pneumonia : Studies have shown that pneumonia in COVID-19 patients is often the triggering factor for PE. However there will be consolidation on chest x-ray, ground glass opacities, peribronchial nodules. There is also history of high grade fever and productive cough in bacterial pneumonia.
Epidemiology and demographics
- Various case reports and case series report relatively high incidence of pulmonary embolism in ICU patients.
- The incidence of thrombotic complications is reported to be 31 % in one study. In this study pulmonary embolism was the most common thrombotic complication..[4]
- Another study reported an overall 24% cumulative incidence of pulmonary embolism in patients with COVID-19 pneumonia, 50% (30–70%) in ICU and 18% (12–27%) in other patients.[5]
- In Non-ICU settings (In-patient), pulmonary embolism is reported to occur in 3% percent of patients in one study.[6]
Risk Factors
Multivariate analysis showed following risk factors that predispose a patient of COVID-19 to pulmonary embolism. [7]
|
Natural History,Complications and Prognosis
Overview
Pulmonary embolism in critically ill patients of COVID-19 is a frequent finding. It can lead to the development of cardiogenic shock, sudden cardiac arrest and pulmonary hypertension if developed chronically. There has been various investigational and treatment approach to treat the hypercoagulability leading to these complications in COVID-19 patients. Patients that are at risk of pulmonary embolism such as those with deep venous thrombosis are advised to take oral anticoagulants. Studies show that there is high cumulative incidence of PE in COVID-19 patients which suggests more frequent use of contrast medium on CT for the evaluation of COVID-19 patients.
Complications
Various complications have been reported ranging from acute right heart failure and sudden cardiac arrest due to pulmonary embolism in COVID-19 patients. The following complications have been frequently reported in various case reports and case series.
Prognosis
COVID-19 patients presenting with pulmonary embolism have a poor prognosis. It has been reported that despite adequate anticoagulation being advised to patients in ICU, there is still relatively high incidence of PE in these patients. Few studies showed VTE to be the main cause of death in COVID-19 patients which suggests setting a lower threshold for diagnostic imaging for DVT or PE.
Diagnosis
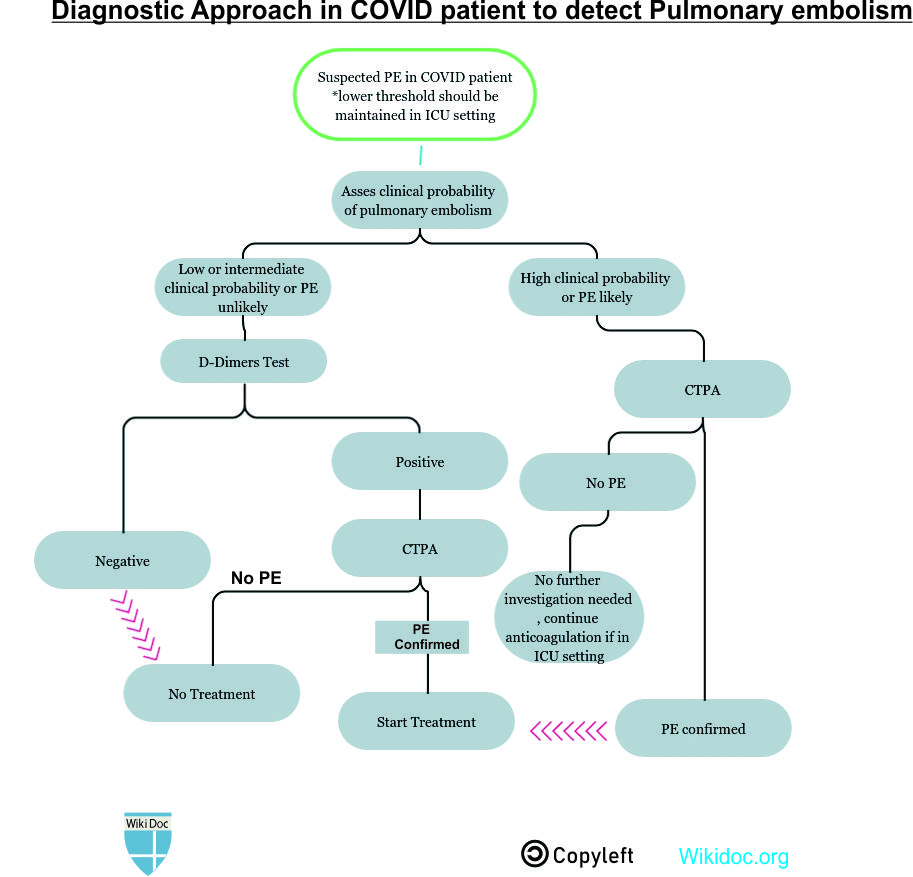
History and Symptoms
- COVID-19 patients are usually at high risk of hypercoagulability and as there is an increased incidence of pulmonary embolism in ICU patients, they mostly have overlapping symptoms with pneumonia, ARDS, and sometimes present only with fever progressing to pulmonary embolism and sudden cardiac arrest.
- Pulmonary embolism can present with no symptoms to shock and even sudden cardiac arrest.
- The most common symptoms that were observed in Prospective Investigation of Pulmonary Embolism Diagnosis II (PIOPED II) trial include:[8]
- Dyspnea that is sudden in onset at rest or exertion (73%)
- Pleuritic pain (44%)
- Calf or thigh pain (44%)
- Calf or thigh swelling (41%)
- Cough (34%)
Physical Examination
On physical examination following signs can be demonstrated in COVID-19 patients.[8]
General
- Tachypnea (>20/min)
- Tachycardia (>100/min)
- Diaphoresis
- Cyanosis
- Temperature > 38.5oC (>101.3oF)
Cardiac examination
- Increased P2
- Right ventricular lift
- Jugular venous distension
Lung examination
- Rales (crackles)
- Wheezes
- Rhonchi
- Decreased breath sounds
- Pleural friction rub
DVT signs
- Calf or thigh
- Calf and thigh
Laboratory findings
Lab findings of different case studies of patients having pulmonary embolism due to COVID-19 are given as [9]
- Elevated d-dimers
- Elevated prothrombin time
- Elevated CRP
- Elevated Cardiac biomarkers
- High fibrinogen
- Mild thrombocytopenia or thrombocytosis
- Platelet count can be normal
Imaging studies
Chest-X ray
Chest radiography in not a primarily diagnostic test in pulmonary embolism patient. It is neither sensitive and nor specific. Chest-X ray is used to rule out other conditions that can mimic symptoms of pulmonary embolism such as bacterial pneumonia ,cardiogenic cause of dyspnea and pneumothorax.
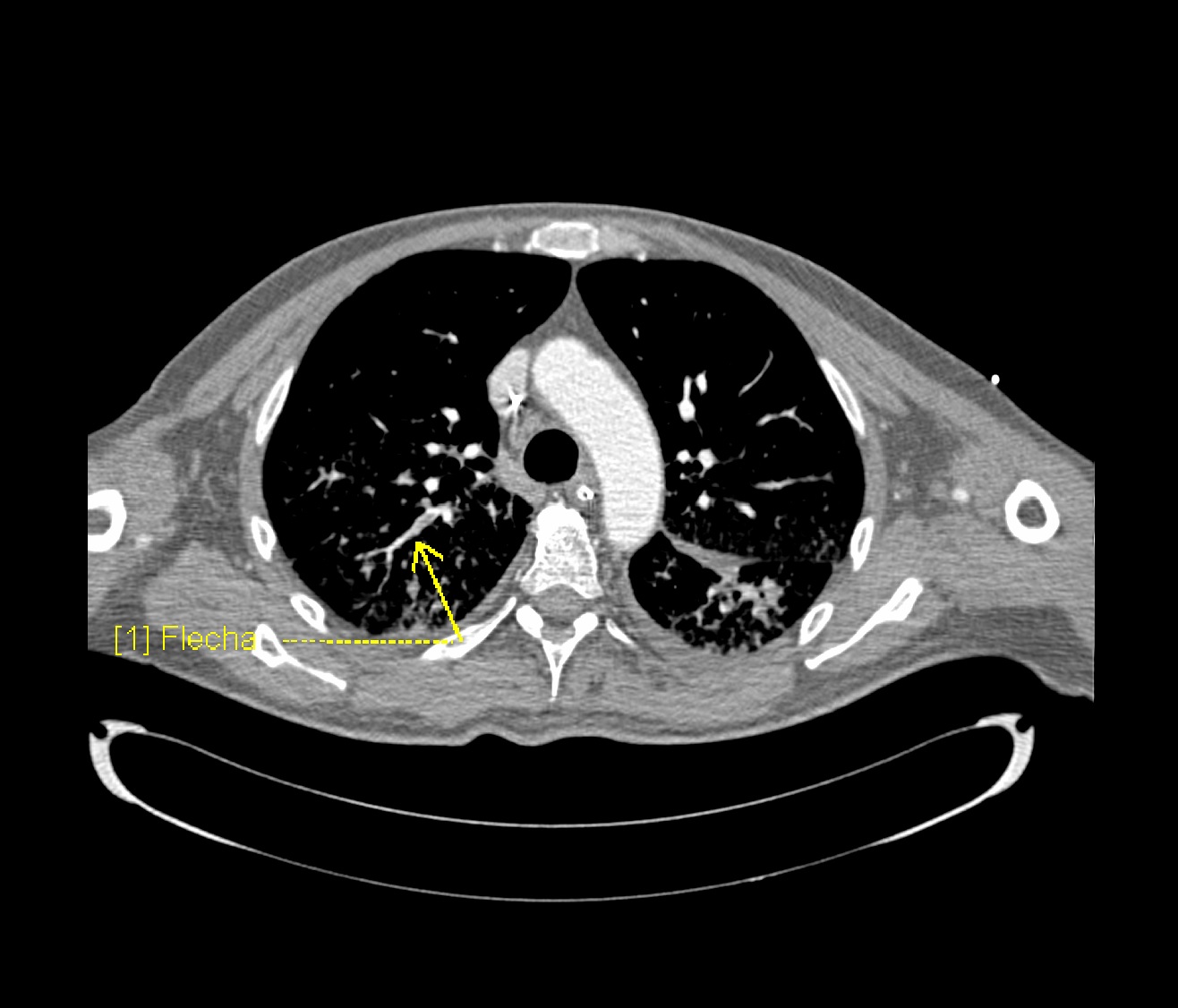
CTPA & Ventilation Perfusion Scan
- Prompt diagnosis of PE in COVID-19 patient is difficult in this regard that various symptoms of COVID-19 overlap with that of pulmonary embolism. American Society of Hematology provides the following guidelines regarding the diagnosis of pulmonary embolism:
- Normal d-dimers level in a patient with low to moderate pretest probability is sufficient to rule out the diagnosis of PE. D-dimers level is usually elevated in COVID-19 patients. This is not applicable to a patient with a high pretest probability.
- Inpatient with suspected PE with symptoms like hypotension, tachycardia, and sudden drop in oxygen saturation with a high pretest probability of PE, computed tomography with pulmonary angiography is used for the diagnosis. Contraindication to the use of CTPA warrants investigation with ventilation/perfusion scan.
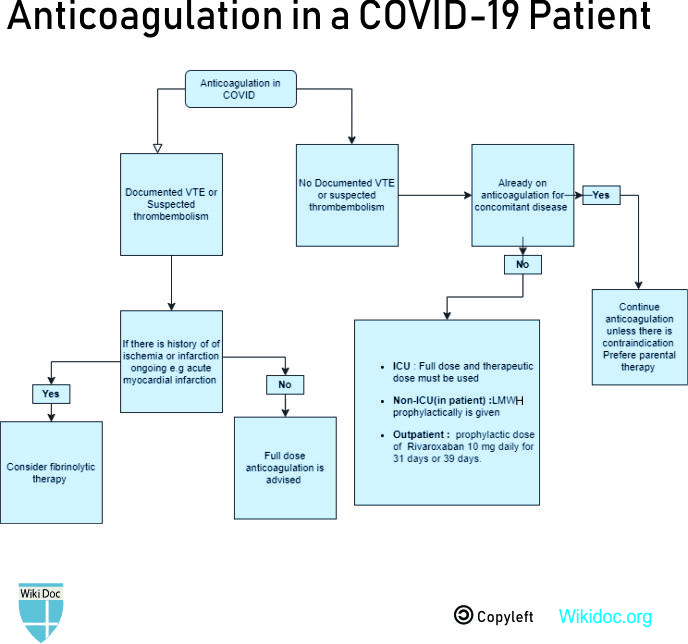
Treatment
Medical Therapy
- Different treatment strategies for COVID-19 patients suffering from pulmonary embolism are given in the table below:
| Different treatment options | Details |
|---|---|
| Prophylaxis | All hospitalized patients with COVID 19 should get proper venous thromboembolism prophylaxis in the absence of any contraindication of anticoagulation.
|
| Acute Pulmonary embolism |
|
| Outpatient treatment[10] |
|
Primary Prevention
The best way to prevent infection is to avoid being exposed to this virus. The following practices should be adopted for infection control:
- Hand washing often with soap and water for at least 20 seconds.
- Using an alcohol-based hand sanitizer that contains at least 60% alcohol if soap and water are not available.
- Avoid touching eyes, nose, and mouth with unwashed hands.
- Avoid close contact with people who are sick
- Stay home when symptomatic
- Cover cough or sneeze with a tissue paper, then throw the tissue in the trash
- Clean and disinfect frequently touched objects and surfaces
There is currently no vaccine to prevent COVID-19.
Secondary Prevention
- Patients not admitted to hospitals but at risk of VTE, such as prior VTE episode, recent surgery, prolonged immobilization are usually given a prophylactic dose of Rivaroxaban 10 mg daily for 31 days or 39 days.
References
- ↑ Wichmann, Dominic; Sperhake, Jan-Peter; Lütgehetmann, Marc; Steurer, Stefan; Edler, Carolin; Heinemann, Axel; Heinrich, Fabian; Mushumba, Herbert; Kniep, Inga; Schröder, Ann Sophie; Burdelski, Christoph; de Heer, Geraldine; Nierhaus, Axel; Frings, Daniel; Pfefferle, Susanne; Becker, Heinrich; Bredereke-Wiedling, Hanns; de Weerth, Andreas; Paschen, Hans-Richard; Sheikhzadeh-Eggers, Sara; Stang, Axel; Schmiedel, Stefan; Bokemeyer, Carsten; Addo, Marylyn M.; Aepfelbacher, Martin; Püschel, Klaus; Kluge, Stefan (2020-05-06). "Autopsy Findings and Venous Thromboembolism in Patients With COVID-19". Annals of Internal Medicine. American College of Physicians. doi:10.7326/m20-2003. ISSN 0003-4819.
- ↑ Teuwen, Laure-Anne; Geldhof, Vincent; Pasut, Alessandra; Carmeliet, Peter (2020-05-21). "COVID-19: the vasculature unleashed". Nature Reviews Immunology. Springer Science and Business Media LLC. doi:10.1038/s41577-020-0343-0. ISSN 1474-1733.
- ↑ Panigada, Mauro; Bottino, Nicola; Tagliabue, Paola; Grasselli, Giacomo; Novembrino, Cristina; Chantarangkul, Veena; Pesenti, Antonio; Peyvandi, Fora; Tripodi, Armando (2020-04-17). "Hypercoagulability of COVID‐19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis". Journal of Thrombosis and Haemostasis. Wiley. doi:10.1111/jth.14850. ISSN 1538-7933.
- ↑ Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; Endeman, H. (2020). "Incidence of thrombotic complications in critically ill ICU patients with COVID-19". Thrombosis Research. Elsevier BV. 191: 145–147. doi:10.1016/j.thromres.2020.04.013. ISSN 0049-3848.
- ↑ Bompard, Florian; Monnier, Hippolyte; Saab, Ines; Tordjman, Mickael; Abdoul, Hendy; Fournier, Laure; Sanchez, Olivier; Lorut, Christine; Chassagnon, Guillaume; Revel, Marie-pierre (2020-05-12). "Pulmonary embolism in patients with Covid-19 pneumonia". European Respiratory Journal. European Respiratory Society (ERS): 2001365. doi:10.1183/13993003.01365-2020. ISSN 0903-1936.
- ↑ Middeldorp, Saskia; Coppens, Michiel; van Haaps, Thijs F.; Foppen, Merijn; Vlaar, Alexander P.; Müller, Marcella C.A.; Bouman, Catherine C.S.; Beenen, Ludo F.M.; Kootte, Ruud S.; Heijmans, Jarom; Smits, Loek P.; Bonta, Peter I.; van Es, Nick (2020-05-05). "Incidence of venous thromboembolism in hospitalized patients with COVID‐19". Journal of Thrombosis and Haemostasis. Wiley. doi:10.1111/jth.14888. ISSN 1538-7933.
- ↑ Poyiadi, Neo; Cormier, Peter; Patel, Parth Y.; Hadied, Mohamad O.; Bhargava, Pallavi; Khanna, Kanika; Nadig, Jeffrey; Keimig, Thomas; Spizarny, David; Reeser, Nicholas; Klochko, Chad; Peterson, Edward L.; Song, Thomas (2020-05-14). "Acute Pulmonary Embolism and COVID-19". Radiology. Radiological Society of North America (RSNA): 201955. doi:10.1148/radiol.2020201955. ISSN 0033-8419.
- ↑ 8.0 8.1 Stein, Paul D.; Beemath, Afzal; Matta, Fadi; Weg, John G.; Yusen, Roger D.; Hales, Charles A.; Hull, Russell D.; Leeper, Kenneth V.; Sostman, H. Dirk; Tapson, Victor F.; Buckley, John D.; Gottschalk, Alexander; Goodman, Lawrence R.; Wakefied, Thomas W.; Woodard, Pamela K. (2007). "Clinical Characteristics of Patients with Acute Pulmonary Embolism: Data from PIOPED II". The American Journal of Medicine. Elsevier BV. 120 (10): 871–879. doi:10.1016/j.amjmed.2007.03.024. ISSN 0002-9343.
- ↑ Bikdeli, Behnood; Madhavan, Mahesh V.; Jimenez, David; Chuich, Taylor; Dreyfus, Isaac; Driggin, Elissa; Nigoghossian, Caroline Der; Ageno, Walter; Madjid, Mohammad; Guo, Yutao; Tang, Liang V.; Hu, Yu; Giri, Jay; Cushman, Mary; Quéré, Isabelle; Dimakakos, Evangelos P.; Gibson, C. Michael; Lippi, Giuseppe; Favaloro, Emmanuel J.; Fareed, Jawed; Caprini, Joseph A.; Tafur, Alfonso J.; Burton, John R.; Francese, Dominic P.; Wang, Elizabeth Y.; Falanga, Anna; McLintock, Claire; Hunt, Beverley J.; Spyropoulos, Alex C.; Barnes, Geoffrey D.; Eikelboom, John W.; Weinberg, Ido; Schulman, Sam; Carrier, Marc; Piazza, Gregory; Beckman, Joshua A.; Steg, P. Gabriel; Stone, Gregg W.; Rosenkranz, Stephan; Goldhaber, Samuel Z.; Parikh, Sahil A.; Monreal, Manuel; Krumholz, Harlan M.; Konstantinides, Stavros V.; Weitz, Jeffrey I.; Lip, Gregory Y.H. (2020). "COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up". Journal of the American College of Cardiology. Elsevier BV. 75 (23): 2950–2973. doi:10.1016/j.jacc.2020.04.031. ISSN 0735-1097.
- ↑