Hypoaldosteronism pathophysiology: Difference between revisions
Akshun Kalia (talk | contribs) No edit summary |
Akshun Kalia (talk | contribs) |
||
| Line 103: | Line 103: | ||
==Associated Conditions== | ==Associated Conditions== | ||
*Sickle cell disease | *[[Sickle-cell disease|Sickle cell disease]] | ||
*Analgesic nephropathy | *[[Analgesic nephropathy]] | ||
*Lead nephropathy | *Lead nephropathy | ||
*Chronic pyelonephritis | *[[Chronic pyelonephritis]] | ||
*Obstructive nephropathy | *[[Obstructive nephropathy]] | ||
*Diabetes and renal insufficiency | *[[Diabetes]] and [[renal insufficiency]] | ||
*Nonsteroidal anti-inflammatory drugs | *[[Nonsteroidal anti-inflammatory drugs]] | ||
*Calcineurin inhibitors | *[[Calcineurin inhibitor|Calcineurin inhibitors]] | ||
*Vitiligo | *[[Vitiligo]] | ||
*Premature ovarian failure | *[[Premature ovarian failure]] | ||
*Pernicious anemia | *[[Pernicious anemia]] | ||
*Myasthenia gravis | *[[Myasthenia gravis]] | ||
*Chronic candidiasis | *Chronic [[candidiasis]] | ||
*Sjögren syndrome | *[[Sjögren's syndrome|Sjögren syndrome]] | ||
*Chronic active hepatitis | *[[Chronic active hepatitis]] | ||
*Hypothyroidism | *[[Hypothyroidism]] | ||
*Graves hyperthyroidism | *[[Graves' disease|Graves]] hyperthyroidism | ||
*Adrenoleukodystrophy | *[[Adrenoleukodystrophy]] | ||
==Gross Pathology== | ==Gross Pathology== | ||
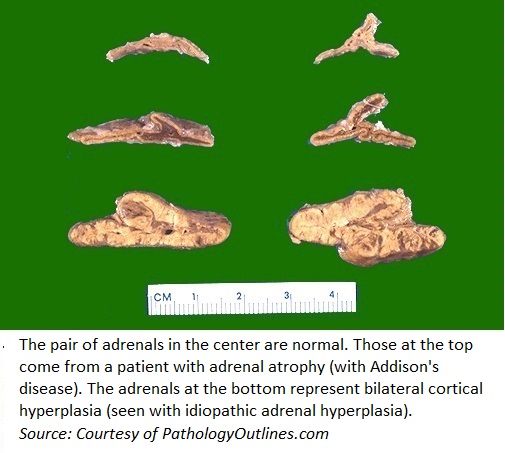
*On gross pathology, adrenal glands in hypoaldosteronism can either be: | *On [[gross pathology]], [[adrenal glands]] in hypoaldosteronism can either be: | ||
**Irregularly shrunken, or | **Irregularly shrunken, or | ||
**Hyperplastic | **[[Hyperplastic]] | ||
[[image:Adrenal glands.jpg|400px|center|Image courtesy: PathologyOutlines.com]] | [[image:Adrenal glands.jpg|400px|center|Image courtesy: PathologyOutlines.com]] | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
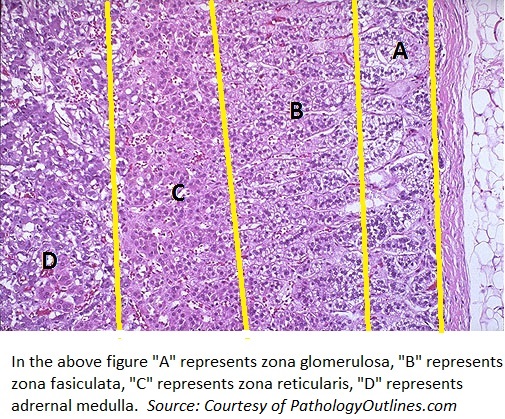
*On microscopic histopathological analysis, adrenal gland can be divided into adrenal cortex and adrenal medulla. | *On [[microscopic]] [[histopathological]] analysis, [[adrenal gland]] can be divided into [[adrenal cortex]] and [[adrenal medulla]]. | ||
*Adrenal cortex can be further categorized into: | *[[Adrenal cortex]] can be further categorized into: | ||
**Zona glomerulosa (produces aldosterone) | **[[Zona glomerulosa]] (produces [[aldosterone]]) | ||
**Zona fasciculata (produces cortisol) | **[[Zona fasciculata]] (produces [[cortisol]]) | ||
**Zona reticularis (produces androgens) | **[[Zona reticularis]] (produces [[androgens]]) | ||
[[image:Adrenal glands normal histology.jpg|400px|center|Image courtesy: PathologyOutlines.com]] | [[image:Adrenal glands normal histology.jpg|400px|center|Image courtesy: PathologyOutlines.com]] | ||
Revision as of 20:37, 30 August 2017
|
Hypoaldosteronism Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Hypoaldosteronism pathophysiology On the Web |
|
American Roentgen Ray Society Images of Hypoaldosteronism pathophysiology |
|
Risk calculators and risk factors for Hypoaldosteronism pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Akshun Kalia M.B.B.S.[2]
Overview
Hypoaldosteronism is defined as decreased levels of the hormone aldosterone or a resistance of the target tissue to the actions of aldosterone. Hypoaldosteronism from decreased production is seen in conditions such as congenital isolated hypoaldosteronism, primary adrenal insufficiency, diabetic nephropathy, critical illness, and drugs such as ACE inhibitors, NSAIDs and calcineurin inhibitors. Resistance of the target tissue to the actions of aldosterone is seen with mineralocorticoid receptor defects (seen in pseudohypoaldosteronism) and with drugs such as potassium-sparing diuretics and trimethoprim. Hypoaldosteronism results in reduced reabsorption of sodium in the principal cells of cortical collecting tubules (CCT). This leads to decreased excretion of potassium (hyperkalemia) and mild non-anion gap metabolic acidosis. On gross pathology, adrenal glands may be irregularly shrunken or hyperplastic.
Pathophysiology
Physiology
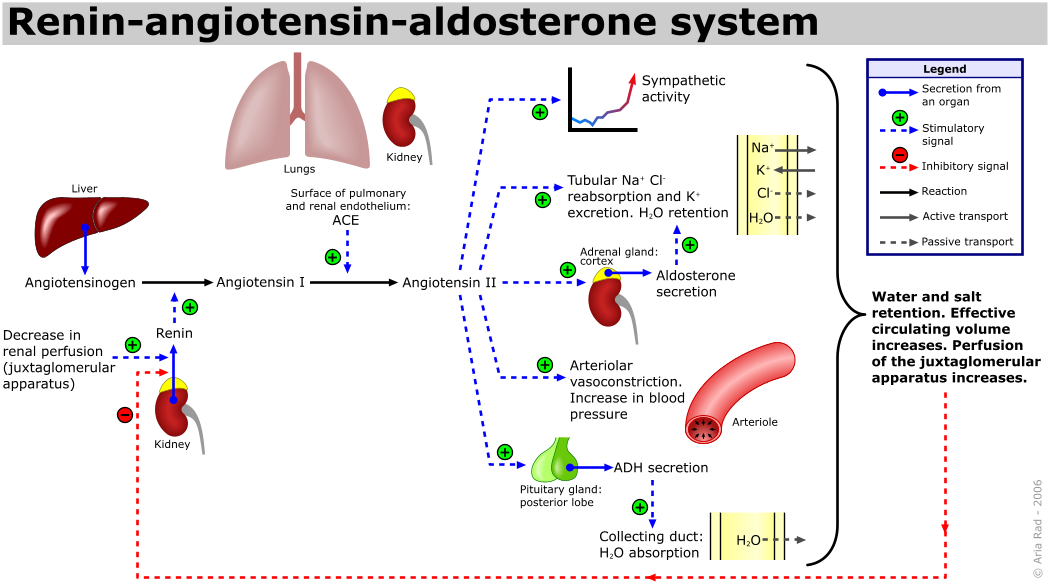
- The juxtaglomerular apparatus of the macula densa primarily senses the concentration of plasma sodium and renal perfusion pressure.
- In response to low plasma sodium concentration or decreased renal perfusion pressure, the juxtaglomerular apparatus secretes renin.
- On secretion, renin cleaves angiotensinogen (produced by liver) to angiotensin I.
- Angiotensin-converting enzyme produced in the lungs further cleaves angiotensin I to angiotensin II.
- The main site of activity for angiotensin II is zona glomerulosa of adrenal cortex, where angiotensin II stimulates aldosterone synthase which converts deoxycorticosterone to aldosterone.
- The renin-angiotensin-aldosterone axis is a tightly controlled feedback mechanism which regulates sodium and blood pressure in our body.
- Low plasma sodium, decreased perfusion pressure and hyperkalemia stimulates aldosterone secretion.
- Hypokalemia suppresses aldosterone secretion.
- Aldosterone is synthesized at a rate of approximately 100 to 150 ug/day.
Pathogenesis
- Hypoaldosteronism is defined as decreased levels of the hormone aldosterone or a resistance of the target tissue to the actions of aldosterone. Hypoaldosteronism can be due to:[1]
- Aldosterone deficiency: The deficiency in aldosterone can be due to congenital isolated hypoaldosteronism, primary adrenal insufficiency, diabetic nephropathy, critical illness, and drugs such as ACE inhibitors, NSAID and calcineurin inhibitors.
- Aldosterone resistance: In aldosterone resistance, the level of aldosterone is normal but there is decreased response of the target tissue to the actions of aldosterone. Aldosterone resistance is seen with mineralocorticoid receptor defects (seen in pseudohypoaldosteronism) and with drugs such as potassium-sparing diuretics and trimethoprim.
- Hypoaldosteronism results in reduced reabsorption of sodium in the principal cells of cortical collecting tubules (CCT). This leads to decreased excretion of potassium and mild non-anion gap metabolic acidosis.
Hyporeninemic Hypoaldosteronism
- Hyporeninemic hypoaldosteronism is most commonly seen in patients with mild to moderate renal insufficiency and diabetic nephropathy:
- In patients of renal insufficiency, atrophy of the juxtaglomerular apparatus (JGA) leads to decreased sensing of plasma sodium concentration and renal perfusion pressure.
- With progression of the renal disease and atrophy of the juxtaglomerular apparatus (JGA) there may be inadequate renin production and release.
- A decrease in renin production and release leads to decreased angiotensin production, which eventually causes hypoaldosteronism .
- Renal insufficiency may also cause decreased response of the principal cells in the cortical collecting tubule to aldosterone.
Hyperreninemic Hypoaldosteronism
- Hyperreninemic hypoaldosteronism also known as secondary isolated hypoaldosteronism is seen in patients with severe illness such as sepsis, malignancy, heart failure, adrenal dysfunction and liver cirrhosis.[2][3][4]
- During these stress inducing conditions, increased levels of ACTH and cortisol are seen.
- Under normal conditions, continuous ACTH secretion for greater than 96 hours leads to suppression of aldosterone synthase activity.
- Chronically ill patients with prolonged ACTH secretion (>96 hours) have impaired aldosterone synthase activity and decreased levels of aldosterone.
- In response, the kidneys via its neurohormonal regulation leads to increased levels of renin and hence the term hyperreninemic hypoaldosteronism.
- In addition, cytokine release from chronic illness or increased levels of atrial natriuretic peptide (in patients with heart failure) also have an inhibitory effect on the zona glomerulosa of adrenal cortex.
- Hyperreninemic hypoaldosteronism is also seen in patients with adrenal dysfunction such as Addison's disease.
- Primary adrenal insufficiency or Addison's disease can be due to adrenal dysgenesis, impaired steroidogenesis, and adrenal destruction.
- In patients of primary adrenal insufficiency, the adrenal glands does not produce sufficient cortisol and aldosterone.
- The decrease in level of aldosterone leads to decreased absorption of sodium in the kidneys and increased retention of potassium. This in turn activates the juxtaglomerular apparatus (JGA) of the kidneys, which secretes renin in an attempt to normalise plasma sodium concentration and perfusion pressure. However, due to adrenal dysfunction the zona glomerulosa of adrenal cortex is unable to produce aldosterone and presents with hyperreninemic hypoaldosteronism.
Isolated Hypoaldosteronism
- In isolated hypoaldosteronism, there is selective deficiency of aldosterone with normal cortisol production. Isolated hypoaldosteronism may result from dysfunction of zona glomerulosa or aldosterone synthase deficiency.
- Aldosterone synthase is an enzyme involved in the synthesis of aldosterone. Patients with aldosterone synthase enzyme deficiency (type I and type II) results in defective conversion of deoxycorticosterone to aldosterone and subsequently abnormal levels of aldosterone.
- Certain drugs such as heparin and nitric oxide have a direct suppressive effect on the zona glomerulosa of adrenal cortex which may lead to decrease production of aldosterone.
Postadrenalectomy Hypoaldosteronism
Postadrenalectomy hypoaldosteronism is seen in patients with Conn syndrome who undergo surgery for tumor removal:[5][6][7][8][9][10][11]
- Conn syndrome is most often unilateral and leads to excessive production of aldosterone from the affected adrenal gland.
- Excessive production of aldosterone causes hypertension and suppression of renin angiotensin aldosterone system (RAAS).
- Patients of Conn syndrome who are treated with spironolactone and later undergo surgery for tumor removal may develop hypoaldosteronism.
- Patients of Conn syndrome have increased levels of aldosterone and decreased plasma renin activity (from suppressed RAAS) which leads to chronic suppression of contralateral zona glomerulosa.
- On surgical removal of aldosterone producing tumor, there is sudden decline in circulating aldosterone which leads to hypoaldosteronism.
Mineralocorticoid Resistance
Mineralocorticoid resistance is characterized by a decrease in response to the hormone aldosterone. In mineralocorticoid resistance the level of aldosterone may be normal or supranormal. It is due to this reason mineralocorticoid resistance is also known as pseudohypoaldosteronism. Mineralocorticoid resistance can be further categorized into:[12][13][14]
- Pseudohypoaldosteronism type I:
- The decrease in response to aldosterone is due to heterozygous or homozygous inactivating mutations in the mineralocorticoid receptor. These patients are also resistant to mineralocorticoid therapy.
- Pseudohypoaldosteronism Type II:
- This is an extremely rare disorder. It is speculated that these patients have mutations in the genes encoding proteins of the serine threonine kinase family (WKNK1 or WNK4 kinases). Pseudohypoaldosteronism Type II is characterized by low or low-normal plasma renin activity and aldosterone concentrations, leading to hyperkalemia and metabolic acidosis with normal renal function. Pseudohypoaldosteronism type II is also known as Gordon’s syndrome.
- Pseudohypoaldosteronism type III:
Genetics
- Genes involved in the pathogenesis of hypoaldosteronism include mutation in CYP11B2 gene and NR3C2 gene.
- CYP11B2 gene is located on chromosome 8q24.
- Mutation in CYP11B2 gene is transmitted in autosomal recessive pattern.
- The CYP11B2 gene encodes for the enzyme aldosterone synthase (previously known as corticosterone methyloxidase).
- Aldosterone synthase catalyses the conversion of 11 deoxycorticosterone to aldosterone. The following flowchart depicts the various steps carried out by the enzyme aldosterone synthase:
| 11 Deoxycorticosterone | |||||||||||||||||||
| Corticosterone | |||||||||||||||||||
| 18 Hydroxycorticosterone | |||||||||||||||||||
| Aldosterone | |||||||||||||||||||
- Mutations in CYP11B2 can lead to:
- Type 1 aldosterone synthase deficiency: Patients have normal to decreased levels of 18-hydroxycorticosterone and undetectable levels of aldosterone.
- Type 2 aldosterone synthase deficiency: Patients have increased levels of 18-hydroxycorticosterone and normal to decreased levels of aldosterone.
- Aldosterone synthase is a member of the cytochrome P450 family of enzymes.
- The other gene associated with hypoaldosteronism is NR3C2 gene.
- NR3C2 gene is located on chromosome 4q31.1-31.2; which is the long (q) arm of chromosome 4 at position 31.1-31.2
- NR3C2 gene encodes for mineralocorticoid receptor.
- Mutation in NR3C2 gene can be transmitted in autosomal recessive or autosomal dominant pattern.
- Mutated NR3C2 gene leads to defective mineralocorticoid receptor and presents with resistance to the actions of aldosterone.
Associated Conditions
- Sickle cell disease
- Analgesic nephropathy
- Lead nephropathy
- Chronic pyelonephritis
- Obstructive nephropathy
- Diabetes and renal insufficiency
- Nonsteroidal anti-inflammatory drugs
- Calcineurin inhibitors
- Vitiligo
- Premature ovarian failure
- Pernicious anemia
- Myasthenia gravis
- Chronic candidiasis
- Sjögren syndrome
- Chronic active hepatitis
- Hypothyroidism
- Graves hyperthyroidism
- Adrenoleukodystrophy
Gross Pathology
- On gross pathology, adrenal glands in hypoaldosteronism can either be:
- Irregularly shrunken, or
- Hyperplastic
Microscopic Pathology
- On microscopic histopathological analysis, adrenal gland can be divided into adrenal cortex and adrenal medulla.
- Adrenal cortex can be further categorized into:
- Zona glomerulosa (produces aldosterone)
- Zona fasciculata (produces cortisol)
- Zona reticularis (produces androgens)
References
- ↑ White PC (1994). "Disorders of aldosterone biosynthesis and action". N. Engl. J. Med. 331 (4): 250–8. doi:10.1056/NEJM199407283310408. PMID 8015573.
- ↑ Kater CE, Biglieri EG, Brust N, Chang B, Hirai J (1982). "Regulation of the mineralocorticoid hormones in adrenocortical disorders with adrenocorticotropin excess". Clin Exp Hypertens A. 4 (9–10): 1749–58. PMID 6291814.
- ↑ Aguilera G, Fujita K, Catt KJ (1981). "Mechanisms of inhibition of aldosterone secretion by adrenocorticotropin". Endocrinology. 108 (2): 522–8. doi:10.1210/endo-108-2-522. PMID 6256154.
- ↑ Singer DR, Shirley DG, Markandu ND, Miller MA, Buckley MG, Sugden AL, Sagnella GA, MacGregor GA (1991). "How important are suppression of aldosterone and stimulation of atrial natriuretic peptide secretion in the natriuretic response to an acute sodium load in man?". Clin. Sci. 80 (4): 293–9. PMID 1851063.
- ↑ Kawasaki T, Uezono K, Ueno M, Noda Y, Kumamoto K, Kawano Y, Ogata M, Fukiyama K, Omae T, Bartter FC (1980). "Influence of unilateral adrenalectomy on renin-angiotensin-aldosterone system in primary aldosteronism". Jpn Heart J. 21 (5): 681–92. PMID 7001091.
- ↑ Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, Deinum J (2009). "Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism". Ann. Intern. Med. 151 (5): 329–37. PMID 19721021.
- ↑ Huang WT, Chau T, Wu ST, Lin SH (2010). "Prolonged hyperkalemia following unilateral adrenalectomy for primary hyperaldosteronism". Clin. Nephrol. 73 (5): 392–7. PMID 20420801.
- ↑ Gadallah MF, Kayyas Y, Boules F (1998). "Reversible suppression of the renin-aldosterone axis after unilateral adrenalectomy for adrenal adenoma". Am. J. Kidney Dis. 32 (1): 160–3. PMID 9669438.
- ↑ Biglieri EG, Slaton PE, Silen WS, Galante M, Forsham PH (1966). "Postoperative studies of adrenal function in primary aldosteronism". J. Clin. Endocrinol. Metab. 26 (5): 553–8. doi:10.1210/jcem-26-5-553. PMID 4287160.
- ↑ Groth H, Vetter W, Stimpel M, Greminger P, Tenschert W, Klaiber E, Vetter H (1985). "Adrenalectomy in primary aldosteronism: a long-term follow-up study". Cardiology. 72 Suppl 1: 107–16. PMID 3902226.
- ↑ Yorke E, Stafford S, Holmes D, Sheth S, Melck A (2015). "Aldosterone deficiency after unilateral adrenalectomy for Conn's syndrome: a case report and literature review". Int J Surg Case Rep. 7C: 141–4. doi:10.1016/j.ijscr.2015.01.013. PMC 4336421. PMID 25604311.
- ↑ CHEEK DB, PERRY JW (1958). "A salt wasting syndrome in infancy". Arch. Dis. Child. 33 (169): 252–6. PMC 2012226. PMID 13545877.
- ↑ Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001). "Human hypertension caused by mutations in WNK kinases". Science. 293 (5532): 1107–12. doi:10.1126/science.1062844. PMID 11498583.
- ↑ Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S (2013). "Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension". Cell Rep. 3 (3): 858–68. doi:10.1016/j.celrep.2013.02.024. PMID 23453970.