Nevirapine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LIFE THREATENING (INCLUDING FATAL) HEPATOTOXICITY AND SKIN REACTIONS
See full prescribing information for complete Boxed Warning.
HEPATOTOXICITY: Severe, life threatening and in some cases fatal hepatotoxicity, particularly in the first 18 weeks, has been reported in patients treated with nevirapine. In some cases, patients presented with non-specific prodromal signs or symptoms of hepatitis and progressed to hepatic failure. These events are often associated with rash. Female gender and higher CD4+ cell counts at initiation of therapy place patients at increased risk; women with CD4+ cell counts greater than 250 cells/mm3, including pregnant women receiving nevirapine in combination with other antiretrovirals for the treatment of HIV-1 infection, are at the greatest risk. However, hepatotoxicity associated with nevirapine use can occur in both genders, all CD4+ cell counts and at any time during treatment. Hepatic failure has also been reported in patients without HIV taking nevirapine for post-exposure prophylaxis (PEP). Use of nevirapine for occupational and non-occupational PEP is contraindicated. Patients with signs or symptoms of hepatitis or with increased transaminases combined with rash or other systemic symptoms, must discontinue nevirapine and seek medical evaluation immediately.
SKIN REACTIONS: Severe, life threatening skin reactions, including fatal cases, have occurred in patients treated with nevirapine. These have included cases of Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity reactions characterized by rash, constitutional findings and organ dysfunction. Patients developing signs or symptoms of severe skin reactions or hypersensitivity reactions must discontinue nevirapine and seek medical evaluation immediately. Transaminase levels should be checked immediately for all patients who develop a rash in the first 18 weeks of treatment. The 14-day lead-in period with nevirapine 200 mg daily dosing has been observed to decrease the incidence of rash and must be followed. MONITORING: Patients must be monitored intensively during the first 18 weeks of therapy with nevirapine to detect potentially life threatening hepatotoxicity or skin reactions. Extra vigilance is warranted during the first 6 weeks of therapy, which is the period of greatest risk of these events. Do not restart nevirapine following clinical hepatitis or transaminase elevations combined with rash or other systemic symptoms or following severe skin rash or hypersensitivity reactions. In some cases, hepatic injury has progressed despite discontinuation of treatment. |
Overview
Nevirapine is a non-nucleoside reverse transcriptase inhibitor that is FDA approved for the treatment of HIV-1 infection. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash and myalgia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Nevirapine tablets are indicated for use in combination with other antiretroviral agents for the treatment of HIV-1 infection.
- Dosage:
- One 200 mg tablet daily for the first 14 days, followed by one 200 mg tablet twice daily, in combination with other antiretroviral agents.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nevirapine in adult patients.
Non–Guideline-Supported Use
- Prophylaxis of perinatal VIH infection
- Prophylaxis of post natal VIH infection
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Nevirapine tablets are indicated for use in combination with other antiretroviral agents for the treatment of HIV-1 infection.
- Dosage for pediatric patients 15 days and older:
- 150 mg/m2 once daily for 14 days followed by 150 mg/m2 twice daily thereafter.
- The total daily dose should not exceed 400 mg for any patient.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nevirapine in pediatric patients.
Non–Guideline-Supported Use
- Prophylaxis of perinatal VIH infection
- Prophylaxis of post natal VIH infection
Contraindications
Hepatic Impairment
- Nevirapine tablets are contraindicated in patients with moderate or severe (Child-Pugh Class B or C, respectively) hepatic impairment.
Post-exposure Prophylaxis
- Nevirapine is contraindicated for use as part of occupational and non-occupational post-exposure prophylaxis (PEP) regimens.
Warnings
|
WARNING: LIFE THREATENING (INCLUDING FATAL) HEPATOTOXICITY AND SKIN REACTIONS
See full prescribing information for complete Boxed Warning.
HEPATOTOXICITY: Severe, life threatening and in some cases fatal hepatotoxicity, particularly in the first 18 weeks, has been reported in patients treated with nevirapine. In some cases, patients presented with non-specific prodromal signs or symptoms of hepatitis and progressed to hepatic failure. These events are often associated with rash. Female gender and higher CD4+ cell counts at initiation of therapy place patients at increased risk; women with CD4+ cell counts greater than 250 cells/mm3, including pregnant women receiving nevirapine in combination with other antiretrovirals for the treatment of HIV-1 infection, are at the greatest risk. However, hepatotoxicity associated with nevirapine use can occur in both genders, all CD4+ cell counts and at any time during treatment. Hepatic failure has also been reported in patients without HIV taking nevirapine for post-exposure prophylaxis (PEP). Use of nevirapine for occupational and non-occupational PEP is contraindicated. Patients with signs or symptoms of hepatitis or with increased transaminases combined with rash or other systemic symptoms, must discontinue nevirapine and seek medical evaluation immediately.
SKIN REACTIONS: Severe, life threatening skin reactions, including fatal cases, have occurred in patients treated with nevirapine. These have included cases of Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity reactions characterized by rash, constitutional findings and organ dysfunction. Patients developing signs or symptoms of severe skin reactions or hypersensitivity reactions must discontinue nevirapine and seek medical evaluation immediately. Transaminase levels should be checked immediately for all patients who develop a rash in the first 18 weeks of treatment. The 14-day lead-in period with nevirapine 200 mg daily dosing has been observed to decrease the incidence of rash and must be followed. MONITORING: Patients must be monitored intensively during the first 18 weeks of therapy with nevirapine to detect potentially life threatening hepatotoxicity or skin reactions. Extra vigilance is warranted during the first 6 weeks of therapy, which is the period of greatest risk of these events. Do not restart nevirapine following clinical hepatitis or transaminase elevations combined with rash or other systemic symptoms or following severe skin rash or hypersensitivity reactions. In some cases, hepatic injury has progressed despite discontinuation of treatment. |
The most serious adverse reactions associated with nevirapine are hepatitis/hepatic failure, Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity reactions. Hepatitis/hepatic failure may be associated with signs of hypersensitivity which can include severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema, eosinophilia, granulocytopenia, lymphadenopathy or renal dysfunction.
The first 18 weeks of therapy with nevirapine are a critical period during which intensive clinical and laboratory monitoring of patients is required to detect potentially life threatening hepatic events and skin reactions. The optimal frequency of monitoring during this time period has not been established. Some experts recommend clinical and laboratory monitoring more often than once per month and in particular, include monitoring of liver enzyme tests at baseline, prior to dose escalation and at 2 weeks post-dose escalation. After the initial 18-week period, frequent clinical and laboratory monitoring should continue throughout nevirapine treatment. In addition, the 14-day lead-in period with nevirapine 200 mg daily dosing has been demonstrated to reduce the frequency of rash.
Hepatotoxicity and Hepatic Impairment
Severe, life threatening and in some cases fatal hepatotoxicity, including fulminant and cholestatic hepatitis, hepatic necrosis and hepatic failure, have been reported in patients treated with nevirapine. In controlled clinical trials, symptomatic hepatic events regardless of severity occurred in 4% (range 0% to 11%) of subjects who received nevirapine and 1% of subjects in control groups.
The risk of symptomatic hepatic events regardless of severity was greatest in the first 6 weeks of therapy. The risk continued to be greater in the nevirapine groups compared to controls through 18 weeks of treatment. However, hepatic events may occur at any time during treatment. In some cases, subjects presented with non-specific, prodromal signs or symptoms of fatigue, malaise, anorexia, nausea, jaundice, liver tenderness or hepatomegaly, with or without initially abnormal serum transaminase levels. Rash was observed in approximately half of the subjects with symptomatic hepatic adverse events. Fever and flu-like symptoms accompanied some of these hepatic events. Some events, particularly those with rash and other symptoms, have progressed to hepatic failure with transaminase elevation, with or without hyperbilirubinemia, hepatic encephalopathy, prolonged partial thromboplastin time or eosinophilia. Rhabdomyolysis has been observed in some patients experiencing skin and/or liver reactions associated with nevirapine use. Patients with signs or symptoms of hepatitis must be advised to discontinue nevirapine and immediately seek medical evaluation, which should include liver enzyme tests.
Transaminases should be checked immediately if a patient experiences signs or symptoms suggestive of hepatitis and/or hypersensitivity reaction. Transaminases should also be checked immediately for all patients who develop a rash in the first 18 weeks of treatment. Physicians and patients should be vigilant for the appearance of signs or symptoms of hepatitis, such as fatigue, malaise, anorexia, nausea, jaundice, bilirubinuria, acholic stools, liver tenderness or hepatomegaly. The diagnosis of hepatotoxicity should be considered in this setting, even if transaminases are initially normal or alternative diagnoses are possible.
If clinical hepatitis or transaminase elevations combined with rash or other systemic symptoms occur, permanently discontinue nevirapine. Do not restart nevirapine after recovery. In some cases, hepatic injury progresses despite discontinuation of treatment.
The patients at greatest risk of hepatic events, including potentially fatal events, are women with high CD4+ cell counts. In general, during the first 6 weeks of treatment, women have a 3-fold higher risk than men for symptomatic, often rash-associated, hepatic events (6% versus 2%) and patients with higher CD4+ cell counts at initiation of nevirapine therapy are at higher risk for symptomatic hepatic events with nevirapine. In a retrospective review, women with CD4+ cell counts greater than 250 cells/mm3 had a 12-fold higher risk of symptomatic hepatic adverse events compared to women with CD4+ cell counts less than 250 cells/mm3 (11% versus 1%). An increased risk was observed in men with CD4+ cell counts greater than 400 cells/mm3 (6% versus 1% for men with CD4+ cell counts less than 400 cells/mm3). However, all patients, regardless of gender, CD4+ cell count or antiretroviral treatment history, should be monitored for hepatotoxicity since symptomatic hepatic adverse events have been reported at all CD4+ cell counts. Co-infection with hepatitis B or C and/or increased transaminase elevations at the start of therapy with nevirapine are associated with a greater risk of later symptomatic events (6 weeks or more after starting nevirapine) and asymptomatic increases in AST or ALT.
In addition, serious hepatotoxicity (including liver failure requiring transplantation in one instance) has been reported in HIV-1 uninfected individuals receiving multiple doses of nevirapine in the setting of post-exposure prophylaxis (PEP), an unapproved use. Use of nevirapine for occupational and non-occupational PEP is contraindicated.
Increased nevirapine trough concentrations have been observed in some patients with hepatic fibrosis or cirrhosis. Therefore, carefully monitor patients with either hepatic fibrosis or cirrhosis for evidence of drug-induced toxicity. Do not administer nevirapine to patients with moderate or severe (Child-Pugh Class B or C, respectively) hepatic impairment.
Skin Reactions
Severe and life threatening skin reactions, including fatal cases, have been reported, occurring most frequently during the first 6 weeks of therapy. These have included cases of Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity reactions characterized by rash, constitutional findings and organ dysfunction including hepatic failure. Rhabdomyolysis has been observed in some patients experiencing skin and/or liver reactions associated with nevirapine use. In controlled clinical trials, Grade 3 and 4 rashes were reported during the first 6 weeks in 2% of nevirapine recipients compared to less than 1% of placebo subjects.
Patients developing signs or symptoms of severe skin reactions or hypersensitivity reactions (including, but not limited to, severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema and/or hepatitis, eosinophilia, granulocytopenia, lymphadenopathy and renal dysfunction) must permanently discontinue nevirapine and seek medical evaluation immediately. Do not restart nevirapine following severe skin rash, skin rash combined with increased transaminases or other symptoms or hypersensitivity reaction.
If patients present with a suspected nevirapine-associated rash, measure transaminases immediately. Permanently discontinue nevirapine in patients with rash-associated transaminase elevations.
Therapy with nevirapine must be initiated with a 14-day lead-in period of 200 mg/day (150 mg/m2/day in pediatric patients), which has been shown to reduce the frequency of rash. Discontinue nevirapine if a patient experiences severe rash or any rash accompanied by constitutional findings. Do not increase nevirapine dose to a patient experiencing a mild to moderate rash without constitutional symptoms during the 14-day lead-in period of 200 mg/day (150 mg/m2/day in pediatric patients) until the rash has resolved. The total duration of the once daily lead-in dosing period must not exceed 28 days at which point an alternative regimen should be sought Patients must be monitored closely if isolated rash of any severity occurs. Delay in stopping nevirapine treatment after the onset of rash may result in a more serious reaction.
Women appear to be at higher risk than men of developing rash with nevirapine.
In a clinical trial, concomitant prednisone use (40 mg/day for the first 14 days of nevirapine administration) was associated with an increase in incidence and severity of rash during the first 6 weeks of nevirapine therapy. Therefore, use of prednisone to prevent nevirapine-associated rash is not recommended.
Resistance
Nevirapine must not be used as a single agent to treat HIV-1 or added on as a sole agent to a failing regimen. Resistant virus emerges rapidly when nevirapine is administered as monotherapy. The choice of new antiretroviral agents to be used in combination with nevirapine should take into consideration the potential for cross resistance. When discontinuing an antiretroviral regimen containing nevirapine, the long half-life of nevirapine should be taken into account; if antiretrovirals with shorter half-lives than nevirapine are stopped concurrently, low plasma concentrations of nevirapine alone may persist for a week or longer and virus resistance may subsequently develop.
Drug Interactions
Concomitant use of St. John's wort (Hypericum perforatum) or St. John's wort-containing products and nevirapine is not recommended. Coadministration of St. John’s wort with non-nucleoside reverse transcriptase inhibitors (NNRTIs), including nevirapine, is expected to substantially decrease NNRTI concentrations and may result in sub-optimal levels of nevirapine and lead to loss of virologic response and possible resistance to nevirapine or to the class of NNRTIs. Coadministration of nevirapine and efavirenz is not recommended as this combination has been associated with an increase in adverse reactions and no improvement in efficacy.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including nevirapine. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia (PCP) or tuberculosis), which may necessitate further evaluation and treatment.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Adverse Reactions
Clinical Trials Experience
Clinical Trials in Adults
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most serious adverse reactions associated with nevirapine are hepatitis, hepatic failure, Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity reactions. Hepatitis/hepatic failure may be isolated or associated with signs of hypersensitivity which may include severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema, eosinophilia, granulocytopenia, lymphadenopathy or renal dysfunction.
Hepatic Reaction
In controlled clinical trials, symptomatic hepatic events regardless of severity occurred in 4% (range 0% to 11%) of subjects who received nevirapine and 1% of subjects in control groups. Female gender and higher CD4+ cell counts (greater than 250 cells/mm3 in women and greater than 400 cells/mm3 in men) place patients at increased risk of these events.
Asymptomatic transaminase elevations (AST or ALT greater than 5X ULN) were observed in 6% (range 0% to 9%) of subjects who received nevirapine and 6% of subjects in control groups. Co-infection with hepatitis B or C and/or increased transaminase elevations at the start of therapy with nevirapine are associated with a greater risk of later symptomatic events (6 weeks or more after starting nevirapine) and asymptomatic increases in AST or ALT.
Liver enzyme abnormalities (AST, ALT, GGT) were observed more frequently in subjects receiving nevirapine than in controls.
Skin Reaction
The most common clinical toxicity of nevirapine is rash, which can be severe or life threatening. Rash occurs most frequently within the first 6 weeks of therapy. Rashes are usually mild to moderate, maculopapular erythematous cutaneous eruptions, with or without pruritus, located on the trunk, face and extremities. In controlled clinical trials (Trials 1037, 1038, 1046 and 1090), Grade 1 and 2 rashes were reported in 13% of subjects receiving nevirapine compared to 6% receiving placebo during the first 6 weeks of therapy. Grade 3 and 4 rashes were reported in 2% of nevirapine recipients compared to less than 1% of subjects receiving placebo. Women tend to be at higher risk for development of nevirapine-associated rash.
Treatment-related, adverse experiences of moderate or severe intensity observed in greater than 2% of subjects receiving nevirapine in placebo-controlled trials are shown in Table 2.

Laboratory Abnormalities
Liver enzyme test abnormalities (AST, ALT) were observed more frequently in subjects receiving nevirapine than in controls. Asymptomatic elevations in GGT occur frequently but are not a contraindication to continue nevirapine therapy in the absence of elevations in other liver enzyme tests. Other laboratory abnormalities (bilirubin, anemia, neutropenia, thrombocytopenia) were observed with similar frequencies in clinical trials comparing nevirapine and control regimens.

Clinical Trials in Pediatric Subjects
Adverse events were assessed in BI Trial 1100.1032 (ACTG 245), a double-blind, placebo-controlled trial of nevirapine (n = 305) in which pediatric subjects received combination treatment with nevirapine. In this trial two subjects were reported to experience Stevens-Johnson syndrome or Stevens-Johnson/toxic epidermal necrolysis transition syndrome. Safety was also assessed in trial BI 1100.882 (ACTG 180), an open-label trial of nevirapine (n = 37) in which subjects were followed for a mean duration of 33.9 months (range: 6.8 months to 5.3 years, including long-term follow-up in 29 of these subjects in trial BI 1100.892). The most frequently reported adverse events related to nevirapine in pediatric subjects were similar to those observed in adults, with the exception of granulocytopenia, which was more commonly observed in children receiving both zidovudine and nevirapine. Cases of allergic reaction, including one case of anaphylaxis, were also reported.
The safety of nevirapine was also examined in BI Trial 1100.1368, an open-label, randomized clinical trial performed in South Africa in which 123 HIV-1 infected treatment-naïve subjects between 3 months and 16 years of age received combination treatment with nevirapine oral suspension, lamuvidine and zidovudine for 48 weeks. Rash (all causality) was reported in 21% of the subjects, four (3%) of whom discontinued drug due to rash. All four subjects experienced the rash early in the course of therapy (less than 4 weeks) and resolved upon nevirapine discontinuation. Other clinically important adverse events (all causality) include neutropenia (9%), anemia (7%) and hepatotoxicity (2%).
Safety information on use of nevirapine in combination therapy in pediatric subjects 2 weeks to less than 3 months of age was assessed in 36 subjects from the BI 1100.1222 (PACTG 356) trial. No unexpected safety findings were observed although granulocytopenia was reported more frequently in this age group compared to the older pediatric age groups and adults.
Postmarketing Experience
In addition to the adverse events identified during clinical trials, the following adverse reactions have been identified during post-approval use of nevirapine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Body as a Whole: fever, somnolence, drug withdrawal, redistribution/accumulation of body fat.
- Gastrointestinal: vomiting
- Liver and Biliary: jaundice, fulminant and cholestatic hepatitis, hepatic necrosis, hepatic failure
- Hematology: anemia, eosinophilia, neutropenia
- Investigations: decreased serum phosphorus
- Musculoskeletal: arthralgia, rhabdomyolysis associated with skin and/or liver reactions
- Neurologic: paraesthesia
- Skin and Appendages: allergic reactions including anaphylaxis, angioedema, bullous eruptions, ulcerative stomatitis and urticaria have all been reported. In addition, hypersensitivity syndrome and hypersensitivity reactions with rash associated with constitutional findings such as fever, blistering, oral lesions, conjunctivitis, facial edema, muscle or joint aches, general malaise, fatigue or significant hepatic abnormalities plus one or more of the following: hepatitis, eosinophilia, granulocytopenia, lymphadenopathy and/or renal dysfunction have been reported.
In post-marketing surveillance anemia has been more commonly observed in children although development of anemia due to concomitant medication use cannot be ruled out.
Drug Interactions
Nevirapine is principally metabolized by the liver via the cytochrome P450 isoenzymes, 3A and 2B6. Nevirapine is known to be an inducer of these enzymes. As a result, drugs that are metabolized by these enzyme systems may have lower than expected plasma levels when coadministered with nevirapine.
The specific pharmacokinetic changes that occur with coadministration of nevirapine and other drugs are listed in Clinical Pharmacology, Table 5. Clinical comments about possible dosage modifications based on established drug interactions are listed in Table 4. The data in Tables 4 and 5 are based on the results of drug interaction trials conducted in HIV-1 seropositive subjects unless otherwise indicated. In addition to established drug interactions, there may be potential pharmacokinetic interactions between nevirapine and other drug classes that are metabolized by the cytochrome P450 system. These potential drug interactions are also listed in Table 4. Although specific drug interaction trials in HIV-1 seropositive subjects have not been conducted for some classes of drugs listed in Table 4, additional clinical monitoring may be warranted when coadministering these drugs.
The in vitro interaction between nevirapine and the antithrombotic agent warfarin is complex. As a result, when giving these drugs concomitantly, plasma warfarin levels may change with the potential for increases in coagulation time. When warfarin is coadministered with nevirapine, anticoagulation levels should be monitored frequently


Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B No observable teratogenicity was detected in reproductive studies performed in pregnant rats and rabbits. The maternal and developmental no-observable-effect level dosages produced systemic exposures approximately equivalent to or approximately 50% higher in rats and rabbits, respectively, than those seen at the recommended daily human dose (based on AUC). In rats, decreased fetal body weights were observed due to administration of a maternally toxic dose (exposures approximately 50% higher than that seen at the recommended human clinical dose).
There are no adequate and well controlled trials of nevirapine in pregnant women. The Antiretroviral Pregnancy Registry, which has been surveying pregnancy outcomes since January 1989, has not found an increased risk of birth defects following first trimester exposures to nevirapine. The prevalence of birth defects after any trimester exposure to nevirapine is comparable to the prevalence observed in the general population.
Severe hepatic events, including fatalities, have been reported in pregnant women receiving chronic nevirapine therapy as part of combination treatment of HIV-1 infection. Regardless of pregnancy status, women with CD4+ cell counts greater than 250 cells/mm3 should not initiate nevirapine unless the benefit outweighs the risk. It is unclear if pregnancy augments the risk observed in non-pregnant women.
Nevirapine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS): B3
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nevirapine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nevirapine during labor and delivery.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-1 infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV-1. Nevirapine is excreted in breast milk. Because of both the potential for HIV-1 transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breast-feed if they are receiving nevirapine.
Pediatric Use
The safety, pharmacokinetic profile and virologic and immunologic responses of nevirapine have been evaluated in HIV-1 infected pediatric subjects age 3 months to 18 years. The safety and pharmacokinetic profile of nevirapine has been evaluated in HIV-1 infected pediatric subjects age 15 days to less than 3 months.
The most frequently reported adverse events related to nevirapine in pediatric subjects were similar to those observed in adults, with the exception of granulocytopenia, which was more commonly observed in children receiving both zidovudine and nevirapine.
Geriatic Use
Clinical trials of nevirapine did not include sufficient numbers of subjects aged 65 and older to determine whether elderly subjects respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy.
Gender
Women appear to be at higher risk than men of developing rash with nevirapine.
Race
An evaluation of nevirapine plasma concentrations (pooled data from several clinical trials) from HIV-1-infected subjects (27 Black, 24 Hispanic, 189 Caucasian) revealed no marked difference in nevirapine steady-state trough concentrations (median Cminss = 4.7 mcg/mL Black, 3.8 mcg/mL Hispanic, 4.3 mcg/mL Caucasian) with long-term nevirapine treatment at 400 mg/day. However, the pharmacokinetics of nevirapine have not been evaluated specifically for the effects of ethnicity.
Renal Impairment
In subjects with renal impairment (mild, moderate or severe), there were no significant changes in the pharmacokinetics of nevirapine. Nevirapine is extensively metabolized by the liver and nevirapine metabolites are extensively eliminated by the kidney. Nevirapine metabolites may accumulate in patients receiving dialysis; however, the clinical significance of this accumulation is not known. No adjustment in nevirapine dosing is required in patients with CrCL greater than or equal to 20 mL/min. In patients undergoing chronic hemodialysis, an additional 200 mg dose following each dialysis treatment is indicated.
Hepatic Impairment
Because increased nevirapine levels and nevirapine accumulation may be observed in patients with serious liver disease, do not administer nevirapine to patients with moderate or severe (Child-Pugh Class B or C, respectively) hepatic impairment.
Females of Reproductive Potential and Males
In reproductive toxicology studies, evidence of impaired fertility was seen in female rats at doses providing systemic exposure, based on AUC, approximately equivalent to that provided with the recommended clinical dose of nevirapine.
Immunocompromised Patients
There is no FDA guidance one the use of Nevirapine in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
Intensive clinical and laboratory monitoring, including liver enzyme tests, is essential at baseline and during the first 18 weeks of treatment with nevirapine tablets. The optimal frequency of monitoring during this period has not been established. Some experts recommend clinical and laboratory monitoring more often than once per month and in particular, would include monitoring of liver enzyme tests at baseline, prior to dose escalation and at two weeks post-dose escalation. After the initial 18-week period, frequent clinical and laboratory monitoring should continue throughout nevirapine treatment. In some cases, hepatic injury has progressed despite discontinuation of treatment.
IV Compatibility
There is limited information regarding the compatibility of Nevirapine and IV administrations.
Overdosage
There is no known antidote for nevirapine overdosage. Cases of nevirapine overdose at doses ranging from 800 mg to 1800 mg per day for up to 15 days have been reported. Patients have experienced events including edema, erythema nodosum, fatigue, fever, headache, insomnia, nausea, pulmonary infiltrates, rash, vertigo, vomiting and weight decrease. All events subsided following discontinuation of nevirapine.
Pharmacology
| |
| Template:Px | |
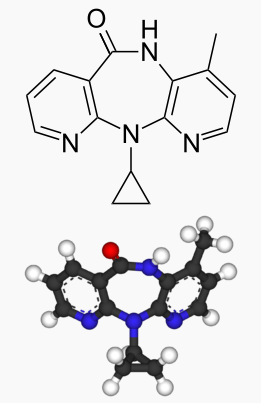
Nevirapine
| |
| Systematic (IUPAC) name | |
| 11-cyclopropyl-4-methyl-5,11-dihydro-6H- dipyrido[3,2-b:2′,3′-e][1,4]diazepin-6-one | |
| Identifiers | |
| CAS number | |
| ATC code | J05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 266.888 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 93% ± 9% |
| Metabolism | Hepatic |
| Half life | 45 hours |
| Excretion | Renal: <6% (Parent drug) Biliary <5% (Parent drug) |
| Therapeutic considerations | |
| Pregnancy cat. |
B: (USA) |
| Legal status | |
| Routes | Oral |
Mechanism of Action
Nevirapine is a non-nucleoside reverse transcriptase inhibitor (NNRTI) of HIV-1. Nevirapine binds directly to reverse transcriptase (RT) and blocks the RNA-dependent and DNA-dependent DNA polymerase activities by causing a disruption of the enzyme's catalytic site. The activity of nevirapine does not compete with template or nucleoside triphosphates. HIV-2 RT and eukaryotic DNA polymerases (such as human DNA polymerases α, ß, γ or δ) are not inhibited by nevirapine.
Structure
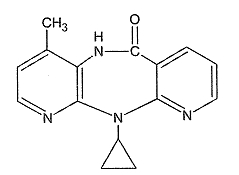
Nevirapine has the following structural formula:
Pharmacodynamics
There is limited information regarding Nevirapine Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption and Bioavailability
Nevirapine is readily absorbed (greater than 90%) after oral administration in healthy volunteers and in adults with HIV-1 infection. Absolute bioavailability in 12 healthy adults following single-dose administration was 93 ± 9% (mean ± SD) for a 50 mg tablet and 91 ± 8% for an oral solution. Peak plasma nevirapine concentrations of 2 ± 0.4 mcg/mL (7.5 micromolar) were attained by 4 hours following a single 200 mg dose. Following multiple doses, nevirapine peak concentrations appear to increase linearly in the dose range of 200 to 400 mg/day. Steady-state trough nevirapine concentrations of 4.5 ± 1.9 mcg/mL (17 ± 7 micromolar), (n = 242) were attained at 400 mg/day. Nevirapine tablets and suspension have been shown to be comparably bioavailable and interchangeable at doses up to 200 mg. When nevirapine (200 mg) was administered to 24 healthy adults (12 female, 12 male), with either a high fat breakfast (857 kcal, 50 g fat, 53% of calories from fat) or antacid (Maalox®** 30 mL), the extent of nevirapine absorption (AUC) was comparable to that observed under fasting conditions. In a separate trial in HIV-1 infected subjects (n = 6), nevirapine steady-state systemic exposure (AUCτ) was not significantly altered by didanosine, which is formulated with an alkaline buffering agent. Nevirapine may be administered with or without food, antacid or didanosine.
Distribution
Nevirapine is highly lipophilic and is essentially nonionized at physiologic pH. Following intravenous administration to healthy adults, the apparent volume of distribution (Vdss) of nevirapine was 1.21 ± 0.09 L/kg, suggesting that nevirapine is widely distributed in humans. Nevirapine readily crosses the placenta and is also found in breast milk. Nevirapine is about 60% bound to plasma proteins in the plasma concentration range of 1 to 10 mcg/mL. Nevirapine concentrations in human cerebrospinal fluid (n = 6) were 45% (± 5%) of the concentrations in plasma; this ratio is approximately equal to the fraction not bound to plasma protein.
Metabolism/Elimination
In vivo trials in humans and in vitro studies with human liver microsomes have shown that nevirapine is extensively biotransformed via cytochrome P450 (oxidative) metabolism to several hydroxylated metabolites. In vitro studies with human liver microsomes suggest that oxidative metabolism of nevirapine is mediated primarily by cytochrome P450 (CYP) isozymes from the CYP3A and CYP2B6 families, although other isozymes may have a secondary role. In a mass balance/excretion trial in eight healthy male volunteers dosed to steady-state with nevirapine 200 mg given twice daily followed by a single 50 mg dose of 14C-nevirapine, approximately 91.4 ± 10.5% of the radiolabeled dose was recovered, with urine (81.3 ± 11.1%) representing the primary route of excretion compared to feces (10.1 ± 1.5%). Greater than 80% of the radioactivity in urine was made up of glucuronide conjugates of hydroxylated metabolites. Thus cytochrome P450 metabolism, glucuronide conjugation and urinary excretion of glucuronidated metabolites represent the primary route of nevirapine biotransformation and elimination in humans. Only a small fraction (less than 5%) of the radioactivity in urine (representing less than 3% of the total dose) was made up of parent compound; therefore, renal excretion plays a minor role in elimination of the parent compound.
Nevirapine is an inducer of hepatic cytochrome P450 (CYP) metabolic enzymes 3A and 2B6. Nevirapine induces CYP3A and CYP2B6 by approximately 20% to 25%, as indicated by erythromycin breath test results and urine metabolites. Autoinduction of CYP3A and CYP2B6 mediated metabolism leads to an approximately 1.5- to 2-fold increase in the apparent oral clearance of nevirapine as treatment continues from a single dose to 2 to 4 weeks of dosing with 200 to 400 mg/day. Autoinduction also results in a corresponding decrease in the terminal phase half-life of nevirapine in plasma, from approximately 45 hours (single dose) to approximately 25 to 30 hours following multiple dosing with 200 to 400 mg/day.
Nonclinical Toxicology
Antiviral Activity
The antiviral activity of nevirapine has been measured in a variety of cell lines including peripheral blood mononuclear cells, monocyte-derived macrophages and lymphoblastoid cell lines. In an assay using human embryonic kidney 293 cells, the median EC50 value (50% inhibitory concentration) of nevirapine was 90 nM against a panel of 2923 isolates of HIV-1 that were primarily (93%) clade B clinical isolates from the United States. The 99th percentile EC50 value was 470 nM in this trial. The median EC50 value was 63 nM (range 14 to 302 nM, n = 29) against clinical isolates of HIV-1 clades A, B, C, D, F, G and H and circulating recombinant forms CRF01_AE, CRF02_AG and CRF12_BF. Nevirapine had no antiviral activity in cell culture against group O HIV-1 isolates (n = 3) or HIV-2 isolates (n = 3) replicating in cord blood mononuclear cells. Nevirapine in combination with efavirenz exhibited strong antagonistic anti-HIV-1 activity in cell culture and was additive to antagonistic with the protease inhibitor ritonavir or the fusion inhibitor enfuvirtide. Nevirapine exhibited additive to synergistic anti-HIV-1 activity in combination with the protease inhibitors amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, saquinavir and tipranavir and the NRTIs abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir and zidovudine. The anti-HIV-1 activity of nevirapine was antagonized by the anti-HBV drug adefovir and by the anti-HCV drug ribavirin in cell culture.
Resistance
HIV-1 isolates with reduced susceptibility (100- to 250-fold) to nevirapine emerge in cell culture. Genotypic analysis showed mutations in the HIV-1 RT gene encoding Y181C and/or V106A substitutions depending upon the virus strain and cell line employed. Time to emergence of nevirapine resistance in cell culture was not altered when selection included nevirapine in combination with several other NNRTIs.
Phenotypic and genotypic changes in HIV-1 isolates from treatment-naïve subjects receiving either nevirapine (n = 24) or nevirapine and ZDV (n = 14) were monitored in Phase 1 and 2 trials over 1 to ≥ 12 weeks. After one week of nevirapine monotherapy, isolates from 3/3 subjects had decreased susceptibility to nevirapine in cell culture. One or more of the RT mutations resulting in amino acid substitutions K103N, V106A, V108I, Y181C, Y188C and G190A were detected in HIV-1 isolates from some subjects as early as 2 weeks after therapy initiation. By week 8 of nevirapine monotherapy, 100% of the subjects tested (n = 24) had HIV-1 isolates with a greater than 100-fold decrease in susceptibility to nevirapine in cell culture compared to baseline and had one or more of the nevirapine-associated RT resistance substitutions. Nineteen of these subjects (80%) had isolates with Y181C substitutions regardless of dose.
Genotypic analysis of isolates from antiretroviral-naïve subjects experiencing virologic failure (n = 71) receiving nevirapine once daily (n = 25) or twice daily (n = 46) in combination with lamivudine and stavudine (trial 2NN) for 48 weeks showed that isolates from 8/25 and 23/46 subjects, respectively, contained one or more of the following NNRTI resistance-associated substitutions: Y181C, K101E, G190A/S, K103N, V106A/M, V108I, Y188C/L, A98G, F227L and M230L.
Cross-resistance
Rapid emergence of HIV-1 strains which are cross-resistant to NNRTIs has been observed in cell culture. Nevirapine-resistant HIV-1 isolates were cross-resistant to the NNRTIs delavirdine and efavirenz. However, nevirapine-resistant isolates were susceptible to the NRTIs ddI and ZDV. Similarly, ZDV-resistant isolates were susceptible to nevirapine in cell culture.
Carcinogenesis and Mutagenesis
Long-term carcinogenicity studies in mice and rats were carried out with nevirapine. Mice were dosed with 0, 50, 375 or 750 mg/kg/day for 2 years. Hepatocellular adenomas and carcinomas were increased at all doses in males and at the two high doses in females. In studies in which rats were administered nevirapine at doses of 0, 3.5, 17.5 or 35 mg/kg/day for 2 years, an increase in hepatocellular adenomas was seen in males at all doses and in females at the high dose. The systemic exposure (based on AUCs) at all doses in the two animal studies was lower than that measured in humans at the 200 mg twice daily dose. The mechanism of the carcinogenic potential is unknown. However, in genetic toxicology assays, nevirapine showed no evidence of mutagenic or clastogenic activity in a battery of in vitro and in vivo studies. These included microbial assays for gene mutation (Ames: Salmonella strains and E. coli), mammalian cell gene mutation assay (CHO/HGPRT), cytogenetic assays using a Chinese hamster ovary cell line and a mouse bone marrow micronucleus assay following oral administration. Given the lack of genotoxic activity of nevirapine, the relevance to humans of hepatocellular neoplasms in nevirapine-treated mice and rats is not known.
Animal Toxicology and/or Pharmacology
Animal studies have shown that nevirapine is widely distributed to nearly all tissues and readily crosses the blood-brain barrier.
Clinical Studies
Clinical Trials in Adults
Trial BI 1090 was a placebo-controlled, double-blind, randomized trial in 2,249 HIV-1 infected subjects with less than 200 CD4+ cells/mm3 at screening. Initiated in 1995, BI 1090 compared treatment with nevirapine + lamivudine + background therapy versus lamivudine + background therapy in NNRTI-naïve subjects. Treatment doses were nevirapine, 200 mg daily for 2 weeks followed by 200 mg twice daily or placebo and lamivudine, 150 mg twice daily. Other antiretroviral agents were given at approved doses. Initial background therapy (in addition to lamivudine) was one NRTI in 1,309 subjects (58%), two or more NRTIs in 771 (34%) and PIs and NRTIs in 169 (8%). The subjects (median age 36.5 years, 70% Caucasian, 79% male) had advanced HIV-1 infection, with a median baseline CD4+ cell count of 96 cells/mm3 and a baseline HIV-1 RNA of 4.58 log10 copies/mL (38,291 copies/mL). Prior to entering the trial, 45% had previously experienced an AIDS-defining clinical event. Eighty-nine percent had antiretroviral treatment prior to entering the trial. BI 1090 was originally designed as a clinical endpoint trial. Prior to unblinding the trial, the primary endpoint was changed to proportion of subjects with HIV-1 RNA less than 50 copies/mL and not previously failed at 48 weeks. Treatment response and outcomes are shown in Table 6.

The change from baseline in CD4+ cell count through one year of therapy was significantly greater for the nevirapine group compared to the placebo group for the overall trial population (64 cells/mm3 vs. 22 cells/mm3, respectively), as well as for subjects who entered the trial as treatment-naïve or having received only ZDV (85 cells/mm3 vs. 25 cells/mm3, respectively).
At 2 years into the trial, 16% of subjects on nevirapine had experienced class C CDC events as compared to 21% of subjects on the control arm.
Trial BI 1046 (INCAS) was a double-blind, placebo-controlled, randomized, three-arm trial with 151 HIV-1 infected subjects with CD4+ cell counts of 200 to 600 cells/mm3 at baseline. BI 1046 compared treatment with nevirapine + zidovudine + didanosine to nevirapine + zidovudine and zidovudine + didanosine. Treatment doses were nevirapine at 200 mg daily for 2 weeks followed by 200 mg twice daily or placebo, zidovudine at 200 mg three times daily and didanosine at 125 mg or 200 mg twice daily (depending on body weight). The subjects had mean baseline HIV-1 RNA of 4.41 log10 copies/mL (25,704 copies/mL) and mean baseline CD4+ cell count of 376 cells/mm3. The primary endpoint was the proportion of subjects with HIV-1 RNA less than 400 copies/mL and not previously failed at 48 weeks. The virologic responder rates at 48 weeks were 45% for subjects treated with nevirapine + zidovudine + didanosine, 19% for subjects treated with zidovudine + didanosine and 0% for subjects treated with nevirapine + zidovudine.
CD4+ cell counts in the nevirapine + ZDV + ddI group increased above baseline by a mean of 139 cells/mm3 at one year, significantly greater than the increase of 87 cells/mm3 in the ZDV + ddI subjects. The nevirapine + ZDV group mean decreased by 6 cells/mm3 below baseline.
Clinical Trials in Pediatric Subjects
The pediatric safety and efficacy of nevirapine was examined in BI Trial 1100.1368, an open-label, randomized clinical trial performed in South Africa in which 123 HIV-1 infected treatment-naïve subjects between 3 months and 16 years of age received nevirapine oral suspension for 48 weeks. Subjects were divided into 4 age groups (3 months to less than 2 years, 2 to less than 7 years, 7 to less than 12 years and 12 to less than or equal to 16 years) and randomized to receive one of two nevirapine doses, determined by 2 different dosing methods [body surface area (150 mg/m2) and weight-based dosing (4 or 7 mg/kg)] in combination with zidovudine and lamivudine. The total daily dose of nevirapine did not exceed 400 mg in either regimen. There were 66 subjects in the body surface area (BSA) dosing group and 57 subjects in the weight-based (BW) dosing group.
Baseline demographics included: 49% male; 81% Black and 19% Caucasian; 4% had previous exposure to ARVs. Subjects had a median baseline HIV-1 RNA of 5.45 log10 copies/mL and a median baseline CD4+ cell count of 527 cells/mm3 (range 37 to 2279). One hundred and five (85%) completed the 48-week period while 18 (15%) discontinued prematurely. Of the subjects who discontinued prematurely, nine (7%) discontinued due to adverse reactions and three (2%) discontinued due to virologic failure. Overall the proportion of subjects who achieved and maintained an HIV-1 RNA less than 400 copies/mL at 48 weeks was 47% (58/123).
How Supplied
Nevirapine 200 mg tablets:
- Bottles of 60 tablets (NDC 0378-4050-91)
- Bottles of 180 tablets (NDC 0378-4050-80)
- Bottles of 500 tablets (NDC 0378-4050-05)
Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Nevirapine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nevirapine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Hepatotoxicity and Skin Reactions
Inform patients of the possibility of severe liver disease or skin reactions associated with nevirapine that may result in death. Instruct patients developing signs or symptoms of liver disease or severe skin reactions to discontinue nevirapine and seek medical attention immediately, including performance of laboratory monitoring. Symptoms of liver disease include fatigue, malaise, anorexia, nausea, jaundice, acholic stools, liver tenderness or hepatomegaly. Symptoms of severe skin or hypersensitivity reactions include rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema and/or hepatitis.
Intensive clinical and laboratory monitoring, including liver enzymes, is essential during the first 18 weeks of therapy with nevirapine to detect potentially life threatening hepatotoxicity and skin reactions. However, liver disease can occur after this period; therefore, monitoring should continue at frequent intervals throughout nevirapine treatment. Extra vigilance is warranted during the first 6 weeks of therapy, which is the period of greatest risk of hepatic events and skin reactions. Advise patients with signs and symptoms of hepatitis to discontinue nevirapine and seek medical evaluation immediately. If nevirapine is discontinued due to hepatotoxicity, do not restart it. Patients, particularly women, with increased CD4+ cell count at initiation of nevirapine therapy (greater than 250 cells/mm3 in women and greater than 400 cells/mm3 in men) are at substantially higher risk for development of symptomatic hepatic events, often associated with rash. Advise patients that co-infection with hepatitis B or C and/or increased transaminases at the start of therapy with nevirapine are associated with a greater risk of later symptomatic events (6 weeks or more after starting nevirapine) and asymptomatic increases in AST or ALT.
The majority of rashes associated with nevirapine occur within the first 6 weeks of initiation of therapy. Instruct patients that if any rash occurs during the 2-week lead-in period, do not escalate the nevirapine dose until the rash resolves. The total duration of the once daily lead-in dosing period should not exceed 28 days, at which point an alternative regimen may need to be started. Any patient experiencing a rash should have their liver enzymes (AST, ALT) evaluated immediately. Patients with severe rash or hypersensitivity reactions should discontinue nevirapine immediately and consult a physician. Nevirapine tablets should not be restarted following severe skin rash or hypersensitivity reaction. Women tend to be at higher risk for development of nevirapine-associated rash.
Administration
Inform patients to take nevirapine tablets every day as prescribed. Patients should not alter the dose without consulting their doctor. If a dose is missed, patients should take the next dose as soon as possible. However, if a dose is skipped, the patient should not double the next dose. Advise patients to report to their doctor the use of any other medications.
Inform patients that it is not known whether nevirapine therapy reduces the risk of transmission of HIV-1 to others through sexual contact. Effective treatment combined with safer sex practices may reduce the chance of passing HIV to others through sexual contact. Patients should be advised to continue to practice safer sex and to use latex or polyurethane condoms to lower the chance of sexual contact with any body fluids such as semen, vaginal secretions or blood. Patients should be advised never to re-use or share needles.
Nevirapine is not a cure for HIV-1 infection; patients may continue to experience illnesses associated with advanced HIV-1 infection, including opportunistic infections. Advise patients to remain under the care of a physician when using nevirapine.
Advise patients taking nevirapine oral suspension to ask their pharmacist for a dosing cup if they do not have one.
Inform patients that they should not take nevirapine tablets or oral suspension and nevirapine extended-release tablets at the same time.
Drug Interactions
Nevirapine may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, non-prescription medication or herbal products, particularly St. John's wort.
Contraceptives
Hormonal methods of birth control, other than depomedroxy-progesterone acetate (DMPA), should not be used as the sole method of contraception in women taking nevirapine, since nevirapine may lower the plasma levels of these medications. Additionally, when oral contraceptives are used for hormonal regulation during nevirapine therapy, the therapeutic effect of the hormonal therapy should be monitored.
Methadone
Nevirapine may decrease plasma concentrations of methadone by increasing its hepatic metabolism. Narcotic withdrawal syndrome has been reported in patients treated with nevirapine and methadone concomitantly. Monitor methadone-maintained patients beginning nevirapine therapy for evidence of withdrawal and adjust methadone dose accordingly.
Fat Redistribution
Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
Precautions with Alcohol
Alcohol-Nevirapine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Viramune [1]
- Viramune O/S
- Viramune XR
Look-Alike Drug Names
There is limited information regarding Nevirapine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Nevirapine |Label Name=Nevirapine 200 mg.png
}}