Tenofovir dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Dosage and Administration
Recommended Dose in Adults and Pediatric Patients 12 Years of Age and Older (35 kg or more)
For the treatment of HIV-1 or chronic hepatitis B: The dose is one 300 mg VIREAD tablet once daily taken orally, without regard to food.
For patients unable to swallow VIREAD tablets, the oral powder formulation (7.5 scoops) may be used.
In the treatment of chronic hepatitis B, the optimal duration of treatment is unknown. Safety and efficacy in pediatric patients with chronic hepatitis B weighing less than 35 kg have not been established.
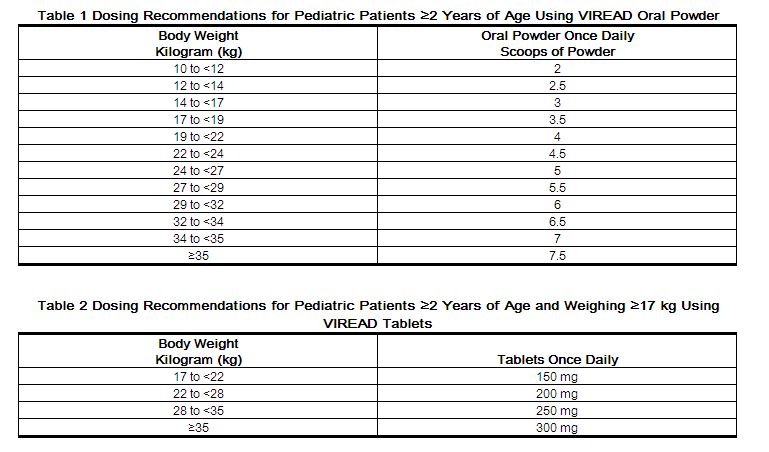
Recommended Dose in Pediatric Patients 2 Years to Less Than 12 Years of Age
HIV-1 Infection
For the treatment of HIV-1 in pediatric patients 2 years of age and older, the recommended oral dose of VIREAD is 8 mg of tenofovir disoproxil fumarate per kilogram of body weight (up to a maximum of 300 mg) once daily administered as oral powder or tablets.
VIREAD oral powder should be measured only with the supplied dosing scoop. One level scoop delivers 1 g of powder which contains 40 mg of tenofovir disoproxil fumarate. VIREAD oral powder should be mixed in a container with 2 to 4 ounces of soft food not requiring chewing (e.g., applesauce, baby food, yogurt). The entire mixture should be ingested immediately to avoid a bitter taste. Do not administer VIREAD oral powder in a liquid as the powder may float on top of the liquid even after stirring. Further patient instructions on how to administer VIREAD oral powder with the supplied dosing scoop are provided in the FDA-approved patient labeling (Patient Information).
VIREAD is also available as tablets in 150, 200, 250 and 300 mg strengths for pediatric patients who weigh greater than or equal to 17 kg and who are able to reliably swallow intact tablets. The dose is one tablet once daily taken orally, without regard to food.
Tables 1 and 2 contain dosing recommendations for VIREAD oral powder and tablets based on body weight. Weight should be monitored periodically and the VIREAD dose adjusted accordingly.
 |
Safety and efficacy of VIREAD in patients younger than 12 years of age have not been established.
Dose Adjustment for Renal Impairment in Adults
Significantly increased drug exposures occurred when VIREAD was administered to subjects with moderate to severe renal impairment [See Clinical Pharmacology (12.3)]. Therefore, the dosing interval of VIREAD tablets 300 mg should be adjusted in patients with baseline creatinine clearance below 50 mL/min using the recommendations in Table 3. These dosing interval recommendations are based on modeling of single-dose pharmacokinetic data in non-HIV and non-HBV infected subjects with varying degrees of renal impairment, including end-stage renal disease requiring hemodialysis. The safety and effectiveness of these dosing interval adjustment recommendations have not been clinically evaluated in patients with moderate or severe renal impairment, therefore clinical response to treatment and renal function should be closely monitored in these patients [See Warnings and Precautions (5.3)]. There are no data to recommend use of VIREAD tablets 150, 200 or 250 mg or VIREAD oral powder in patients with renal impairment.
No dose adjustment of VIREAD tablets 300 mg is necessary for patients with mild renal impairment (creatinine clearance 50–80 mL/min). Routine monitoring of estimated creatinine clearance, serum phosphorus, urine glucose, and urine protein should be performed in patients with mild renal impairment.
 |
The pharmacokinetics of tenofovir have not been evaluated in non-hemodialysis patients with creatinine clearance below 10 mL/min; therefore, no dosing recommendation is available for these patients.
No data are available to make dose recommendations in pediatric patients with renal impairment.[1]
References
Adapted from the FDA Package Insert.