Ribavirin clinical studies
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Clinical Studies
Clinical Study 1 evaluated PegIntron monotherapy. See PegIntron labeling for information about this trial.
REBETOL/PegIntron Combination Therapy
Adult Subjects
Study 2
A randomized trial compared treatment with two PegIntron/REBETOL regimens [PegIntron 1.5 mcg/kg subcutaneously once weekly/REBETOL 800 mg orally daily (in divided doses); PegIntron 1.5 mcg/kg subcutaneously once weekly for 4 weeks then 0.5 mcg/kg subcutaneously once weekly for 44 weeks/REBETOL 1000 or 1200 mg orally daily (in divided doses)] with INTRON A [3 MIU subcutaneously three times weekly/REBETOL 1000 or 1200 mg orally daily (in divided doses)] in 1530 adults with chronic hepatitis C. Interferon-naïve subjects were treated for 48 weeks and followed for 24 weeks post-treatment. Eligible subjects had compensated liver disease, detectable HCV-RNA, elevated ALT, and liver histopathology consistent with chronic hepatitis.
Response to treatment was defined as undetectable HCV-RNA at 24 weeks post-treatment (see Table 13). The response rate to the PegIntron 1.5 mcg/kg and ribavirin 800 mg dose was higher than the response rate to INTRON A/REBETOL (see Table 13).The response rate to PegIntron 1.5→0.5 mcg/kg/REBETOL was essentially the same as the response to INTRON A/REBETOL (data not shown).
 |
Subjects with viral genotype 1, regardless of viral load, had a lower response rate to PegIntron (1.5 mcg/kg)/REBETOL (800 mg) compared to subjects with other viral genotypes. Subjects with both poor prognostic factors (genotype 1 and high viral load) had a response rate of 30% (78/256) compared to a response rate of 29% (71/247) with INTRON A/REBETOL combination therapy.
Subjects with lower body weight tended to have higher adverse-reaction rates [see Adverse Reactions (6.1)] and higher response rates than subjects with higher body weights. Differences in response rates between treatment arms did not substantially vary with body weight.
Treatment response rates with PegIntron/REBETOL combination therapy were 49% in men and 56% in women. Response rates were lower in African American and Hispanic subjects and higher in Asians compared to Caucasians. Although African Americans had a higher proportion of poor prognostic factors compared to Caucasians, the number of non-Caucasians studied (11% of the total) was insufficient to allow meaningful conclusions about differences in response rates after adjusting for prognostic factors in this trial.
Liver biopsies were obtained before and after treatment in 68% of subjects. Compared to baseline, approximately two-thirds of subjects in all treatment groups were observed to have a modest reduction in inflammation.
Study 3
In a large United States community-based trial, 4913 subjects with chronic hepatitis C were randomized to receive PegIntron 1.5 mcg/kg subcutaneously once weekly in combination with a REBETOL dose of 800 to 1400 mg (weight-based dosing [WBD]) or 800 mg (flat) orally daily (in divided doses) for 24 or 48 weeks based on genotype. Response to treatment was defined as undetectable HCV-RNA (based on an assay with a lower limit of detection of 125 IU/mL) at 24 weeks post-treatment.
Treatment with PegIntron 1.5 mcg/kg and REBETOL 800 to 1400 mg resulted in a higher sustained virologic response compared to PegIntron in combination with a flat 800 mg daily dose of REBETOL. Subjects weighing greater than 105 kg obtained the greatest benefit with WBD, although a modest benefit was also observed in subjects weighing greater than 85 to 105 kg (see Table 14). The benefit of WBD in subjects weighing greater than 85 kg was observed with HCV genotypes 1-3. Insufficient data were available to reach conclusions regarding other genotypes. Use of WBD resulted in an increased incidence of anemia [see Adverse Reactions (6.1)].
 |
A total of 1552 subjects weighing greater than 65 kg in Study 3 had genotype 2 or 3 and were randomized to 24 or 48 weeks of therapy. No additional benefit was observed with the longer treatment duration.
Study 4
A large randomized trial compared the safety and efficacy of treatment for 48 weeks with two PegIntron/REBETOL regimens [PegIntron 1.5 mcg/kg and 1 mcg/kg subcutaneously once weekly both in combination with REBETOL 800 to 1400 mg PO daily (in two divided doses)] and Pegasys 180 mcg subcutaneously once weekly in combination with Copegus 1000 to 1200 mg PO daily (in two divided doses) in 3070 treatment-naïve adults with chronic hepatitis C genotype 1. In this trial, lack of early virologic response (undetectable HCV-RNA or greater than or equal to 2 log10 reduction from baseline) by treatment Week 12 was the criterion for discontinuation of treatment. SVR was defined as undetectable HCV-RNA (Roche COBAS TaqMan assay, a lower limit of quantitation of 27 IU/mL) at 24 weeks post-treatment (seeTable 15).
 |
Overall SVR rates were similar among the three treatment groups. Regardless of treatment group, SVR rates were lower in subjects with poor prognostic factors. Subjects with poor prognostic factors randomized to PegIntron (1.5 mcg/kg)/REBETOL or Pegasys/Copegus, however, achieved higher SVR rates compared to similar subjects randomized to PegIntron 1 mcg/kg/REBETOL. For the PegIntron 1.5 mcg/kg and REBETOL dose, SVR rates for subjects with and without the following prognostic factors were as follows: cirrhosis (10% vs. 42%), normal ALT levels (32% vs. 42%), baseline viral load greater than 600,000 IU/mL (35% vs. 61%), 40 years of age and older (38% vs. 50%), and African American race (23% vs. 44%). In subjects with undetectable HCV-RNA at treatment Week 12 who received PegIntron (1.5 mcg/kg)/REBETOL, the SVR rate was 81% (328/407).
Study 5 - REBETOL/PegIntron Combination Therapy in Prior Treatment Failures=
In a noncomparative trial, 2293 subjects with moderate to severe fibrosis who failed previous treatment with combination alpha interferon/ribavirin were re-treated with PegIntron, 1.5 mcg/kg subcutaneously, once weekly, in combination with weight adjusted ribavirin. Eligible subjects included prior nonresponders (subjects who were HCV-RNA positive at the end of a minimum 12 weeks of treatment) and prior relapsers (subjects who were HCV-RNA negative at the end of a minimum 12 weeks of treatment and subsequently relapsed after post-treatment follow-up). Subjects who were negative at Week 12 were treated for 48 weeks and followed for 24 weeks post-treatment. Response to treatment was defined as undetectable HCV-RNA at 24 weeks post-treatment (measured using a research-based test, limit of detection 125 IU/mL). The overall response rate was 22% (497/2293) (99% CI: 19.5, 23.9). Subjects with the following characteristics were less likely to benefit from re-treatment: previous nonresponse, previous pegylated interferon treatment, significant bridging fibrosis or cirrhosis, and genotype 1 infection.
The re-treatment sustained virologic response rates by baseline characteristics are summarized in Table 16.
 |
Achievement of an undetectable HCV-RNA at treatment Week 12 was a strong predictor of SVR. In this trial, 1470 (64%) subjects did not achieve an undetectable HCV-RNA at treatment Week 12, and were offered enrollment into long-term treatment trials, due to an inadequate treatment response. Of the 823 (36%) subjects who were HCV-RNA undetectable at treatment Week 12, those infected with genotype 1 had an SVR of 48% (245/507), with a range of responses by fibrosis scores (F4-F2) of 39-55%. Subjects infected with genotype 2/3 who were HCV-RNA undetectable at treatment Week 12 had an overall SVR of 70% (196/281), with a range of responses by fibrosis scores (F4-F2) of 60-83%. For all genotypes, higher fibrosis scores were associated with a decreased likelihood of achieving SVR.
Pediatric Subjects
Previously untreated pediatric subjects 3 to 17 years of age with compensated chronic hepatitis C and detectable HCV-RNA were treated with REBETOL 15 mg/kg per day and PegIntron 60 mcg/m2 once weekly for 24 or 48 weeks based on HCV genotype and baseline viral load. All subjects were to be followed for 24 weeks post-treatment. A total of 107 subjects received treatment, of which 52% were female, 89% were Caucasian, and 67% were infected with HCV Genotype 1. Subjects infected with Genotypes 1, 4 or Genotype 3 with HCV-RNA greater than or equal to 600,000 IU/mL received 48 weeks of therapy while those infected with Genotype 2 or Genotype 3 with HCV-RNA less than 600,000 IU/mL received 24 weeks of therapy. The trial results are summarized in Table 17.
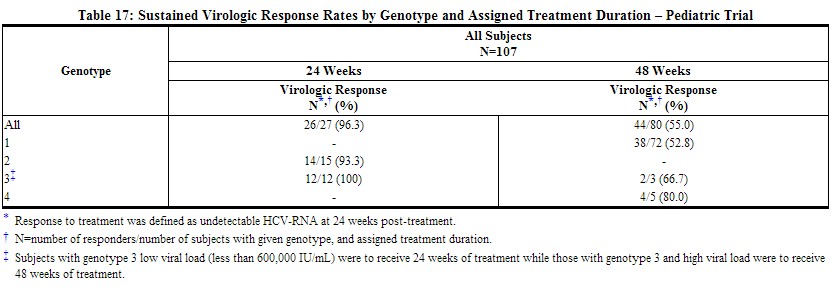 |
REBETOL/INTRON A Combination Therapy
Adult Subjects
Previously Untreated Subjects
Adults with compensated chronic hepatitis C and detectable HCV-RNA (assessed by a central laboratory using a research-based RT-PCR assay) who were previously untreated with alpha interferon therapy were enrolled into two multicenter, double-blind trials (US and international) and randomized to receive REBETOL capsules 1200 mg/day (1000 mg/day for subjects weighing less than or equal to 75 kg) and INTRON A 3 MIU three times weekly or INTRON A and placebo for 24 or 48 weeks followed by 24 weeks of off-therapy follow-up. The international trial did not contain a 24-week INTRON A and placebo treatment arm. The US trial enrolled 912 subjects who, at baseline, were 67% male, 89% Caucasian with a mean Knodell HAI score (I+II+III) of 7.5, and 72% genotype 1. The international trial, conducted in Europe, Israel, Canada, and Australia, enrolled 799 subjects (65% male, 95% Caucasian, mean Knodell score 6.8, and 58% genotype 1).
Trial results are summarized in Table 18.
 |
Of subjects who had not achieved HCV-RNA below the limit of detection of the research-based assay by Week 24 of REBETOL/INTRON A treatment, less than 5% responded to an additional 24 weeks of combination treatment.
Among subjects with HCV Genotype 1 treated with REBETOL/INTRON A therapy who achieved HCV-RNA below the detection limit of the research-based assay by 24 weeks, those randomized to 48 weeks of treatment had higher virologic responses compared to those in the 24-week treatment group. There was no observed increase in response rates for subjects with HCV non-genotype 1 randomized to REBETOL/INTRON A therapy for 48 weeks compared to 24 weeks.
Relapse Subjects
Subjects with compensated chronic hepatitis C and detectable HCV-RNA (assessed by a central laboratory using a research-based RT-PCR assay) who had relapsed following one or two courses of interferon therapy (defined as abnormal serum ALT levels) were enrolled into two multicenter, double-blind trials (US and international) and randomized to receive REBETOL 1200 mg/day (1000 mg/day for subjects weighing ≤75 kg) and INTRON A 3 MIU three times weekly or INTRON A and placebo for 24 weeks followed by 24 weeks of off-therapy follow-up. The US trial enrolled 153 subjects who, at baseline, were 67% male, 92% Caucasian with a mean Knodell HAI score (I+II+III) of 6.8, and 58% genotype 1. The international trial, conducted in Europe, Israel, Canada, and Australia, enrolled 192 subjects (64% male, 95% Caucasian, mean Knodell score 6.6, and 56% genotype 1). Trial results are summarized in Table 19.
 |
Virologic and histologic responses were similar among male and female subjects in both the previously untreated and relapse trials.
Pediatric Subjects
Pediatric subjects 3 to 16 years of age with compensated chronic hepatitis C and detectable HCV-RNA (assessed by a central laboratory using a research-based RT-PCR assay) were treated with REBETOL 15 mg/kg per day and INTRON A 3 MIU/m2 three times weekly for 48 weeks followed by 24 weeks of off-therapy follow-up. A total of 118 subjects received treatment, of which 57% were male, 80% Caucasian, and 78% genotype 1. Subjects less than 5 years of age received REBETOL oral solution and those 5 years of age or older received either REBETOL oral solution or capsules.
Trial results are summarized in Table 20.
 |
Subjects with viral genotype 1, regardless of viral load, had a lower response rate to INTRON A/REBETOL combination therapy compared to subjects with genotype non-1, 36% vs. 81%. Subjects with both poor prognostic factors (genotype 1 and high viral load) had a response rate of 26% (13/50).[1]
References
- ↑ "REBETOL (RIBAVIRIN) CAPSULE REBETOL (RIBAVIRIN) LIQUID [MERCK SHARP & DOHME CORP.]". Retrieved 6 January 2014.
Adapted from the FDA Package Insert.