Phosphate reserves
A well-fed adult in the industrialized world consumes and excretes about 1-3 g of phosphorus per day in the form of phosphate (2-6 x 1022 molecules). Per the elemental composition of the "standard man" of 70 kg, phosphorus is 780 g or 1.1% (as 1.52 x 1025 molecules of phosphate).[1] Of this 1.4 g/kg (98 g, 1.9 x 1024 molecules of phosphate) are present in soft tissue with the remainder (1.33 x 1025 molecules of phosphate) in mineralized tissue such as bone and teeth.[2] Only about 0.1% of body phosphate (about 2 x 1022 molecules) circulates in the blood, but this amount reflects the amount of phosphate available to soft tissue cells. Blood plasma contains orthophosphate (as HPO42-) and H2PO4- in the ratio of about 4:1.[2]
The total quantity of ATP in the human body is about 0.1 mole (about 6 x 1022 molecules). This ATP is constantly being broken down into ADP, and then converted back into ATP. At any given time, the total amount of ATP + ADP remains fairly constant. The energy used by human cells requires the hydrolysis of 100 to 150 moles (6 to 9 x 1025 molecules) of ATP daily which is around 50 to 75 kg. Typically, a human will use up their body weight of ATP over the course of the day.[3] This means that each ATP molecule is recycled 1000 to 1500 times daily, or about once every minute.
There are intracellular and extracellular PPi levels in many tissues.
Intracellular phosphate
With the number of cells in the human body of 10-100 trillion or 1013 to 1014, there are approximately 1.9-19.0 x 1010 atoms of phosphorus (1.9 to 19 x 1010 molecules of phosphate) per cell. Some of this, ~6 x 108 molecules can be ATP.
A typical cell volume is 5 x 10-16 m3. If a typical cell was totally liquid water, there would be about 1.7 x 1013 molecules of water present. Then the phosphate concentration would be on the order of 10-3. However, a cell is about twice as dense as liquid water, then perhaps only 50% water yielding 5 x 10-3 for phosphate concentration. As computer simulation has shown the probability of finding a target by diffusion decreases rapidly with distance and becomes <1% when the starting distance exceeds the target's 10-fold radius,[4] which by assumption of a simple spherical volume would put the target's concentration on the order of 10-3.
Although ectonucleotide pyrophosphatase/phosphodiesterase 3 (ENPP3) regulates intracellular PPi concentrations it does not seem to significantly regulate extracellular PPi.[5]
Extracellular phosphate
PPi inhibits hydroxyapatite deposition in bone and cartilage.[5] Many studies have shown that PPi is a potent inhibitor of calcification, bone mineralization, and bone resorption.[6] Human defects in alkaline phosphatase, an enzyme that degrades PPi, lead to an increase in PPi levels and a severe block in skeletal mineralization.[6] Genetic defects in a cell surface ectoenzyme that normally generates extracellular PPi from nucleotide triphosphate cause ectopic mineralization of joints and ligaments and may be associated with spinal ligament ossification in humans.[6]
Teeth
Enamel is the hardest and most highly mineralized substance of the body and is one of the four major tissues which make up the tooth, along with dentin, cementum, and dental pulp.[7] Ninety-six percent of enamel consists of mineral, with water and organic material composing the rest.[8] Enamel varies in thickness over the surface of the tooth and is often thickest at the cusp, up to 2.5 mm, and thinnest at its border, which is seen clinically as the cementoenamel junction (CEJ).[9] Enamel's primary mineral is hydroxyapatite, which is a crystalline calcium phosphate.[10] Unlike dentin and bone, enamel does not contain collagen. Instead, it has two unique classes of proteins called amelogenins and enamelins.
The basic unit of enamel is called an enamel rod.[10] Measuring 4 μm - 8 μm in diameter an enamel rod, formerly called an enamel prism, is a tightly packed mass of hydroxyapatite crystals in an organized pattern.[7] In cross section, it is best compared to a keyhole, with the top, or head, oriented toward the crown of the tooth, and the bottom, or tail, oriented toward the root of the tooth.
The arrangement of the crystals within each enamel rod is highly complex. Both ameloblasts (the cells which initiate enamel formation) and Tomes' processes affect the crystals' pattern. Enamel crystals in the head of the enamel rod are oriented parallel to the long axis of the rod.[9][7] When found in the tail of the enamel rod, the crystals' orientation diverges slightly from the long axis.[7]
Dentin is the substance between enamel or cementum and the pulp chamber. Dentin, which is less mineralized and less brittle, compensates for enamel and is necessary as a support.[10] The porous, yellow-hued material is made up of 70% by weight, mainly the mineral, hydroxylapatite and some non-crystalline amorphous calcium phosphate), 20% organic materials (90% of which is collagen type 1 and the remaining 10% ground substance, which includes dentine-specific proteins), and 10% water by weight.[11] Dentin is a mineralized connective tissue with an organic matrix of collagenous proteins.
Cementum is a specialized bony substance covering the root of a tooth.[12] It is approximately 45% inorganic material (mainly hydroxyapatite), 33% organic material (mainly collagen) and 22% water. The chemical makeup of cementum is similar to that of bone, but it lacks vascularization. Volumetrically, it is approximately 65% inorganic material (mainly hydroxyapatite), 23% organic material (mainly collagen type1]) and 12% water.
Pulp is the part in the center of a tooth made up of living soft tissue and cells called odontoblasts. Each person has a total of 52 pulp organs, 32 in the permanent and 20 in the primary teeth. The total volume of all the permanent teeth organs is 0.38cc and the mean volume of a single adult human pulp is 0.02cc.
Bone
The matrix is the major constituent of bone, surrounding the cells. It has inorganic and organic parts. Bone is not a uniformly solid material, but rather has some spaces between its hard elements.
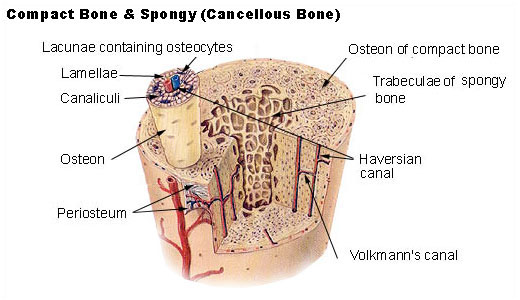
Compact bone
The hard outer layer of bones is composed of compact bone tissue, so-called due to its minimal gaps and spaces. This tissue gives bones their smooth, white, and solid appearance, and accounts for 80% of the total bone mass of an adult skeleton. Compact bone may also be referred to as dense bone or cortical bone.
Trabecular bone
Filling the interior of the organ is the trabecular bone tissue (an open cell porous network also called cancellous or spongy bone) which is comprised of a network of rod- and plate-like elements that make the overall organ lighter and allowing room for blood vessels and marrow. Trabecular bone accounts for the remaining 20% of total bone mass, but has nearly ten times the surface area of compact bone.
Inorganic matrix
The inorganic matrix is mainly crystalline mineral salts and calcium, which is present in the form of hydroxyapatite. The matrix is initially laid down as unmineralized osteoid (manufactured by osteoblasts). Mineralisation involves osteoblasts secreting vesicles containing alkaline phosphatase. This cleaves the phosphate groups and acts as the foci for calcium and phosphate deposition. The vesicles then rupture and act as a centre for crystals to grow on.
Organic matrix
The organic part of matrix is mainly Type I collagen. This is made intracellularly as tropocollagen and then exported. It then associates into fibrils. Also making up the organic part of matrix include various growth factors, the functions of which are not fully known. Other factors present include glycosaminoglycans, osteocalcin, osteonectin, bone sialo protein and Cell Attachment Factor. One of the main things that distinguishes the matrix of a bone from that of another cell is that the matrix in bone is hard.
Woven or lamellar matrix

Bone is first deposited as woven bone, in a disorganized structure with a high proportion of osteocytes in young and in healing injuries. Woven bone is weaker, with a small number of randomly oriented collagen fibers, but forms quickly. It is replaced by lamellar bone, which is highly organized in concentric sheets with a low proportion of osteocytes. Lamellar bone is stronger and filled with many collagen fibers parallel to other fibers in the same layer. The fibers run in opposite directions in alternating layers, much like plywood, assisting in the bone's ability to resist torsion forces. After a break, woven bone quickly forms and is gradually replaced by slow-growing lamellar bone on pre-existing calcified hyaline cartilage through a process known as "bony substitution."
Bone resorption
During bone resorption high levels of phosphate are released into the ECF as osteoclasts tunnel into mineralized bone, breaking it down and releasing phosphate, that results in a transfer of phosphate from bone fluid to the blood. During childhood, bone formation exceeds resorption, but as the aging process occurs, resorption exceeds formation.
Phosphate molecule locations
| Type | Form | Function | Distribution | Molecules | |
|---|---|---|---|---|---|
| solute | ATP, molecule | energy, structure | average cell | ~6 x 108 | |
| solute | molecule | energy, structure | average cell | 1.9 to 19 x 1010 | |
| solute | ions, molecules | availability | blood | ~2 x 1022[2] | |
| mineral | calcium phosphate | enamel | teeth | ~2.49 x 1022[13] | |
| solute | molecule | energy, structure | human body | ~6 x 1022 | |
| all | all | all | soft tissue | 1.9 x 1024[2] | |
| mineral | calcium phosphate | all | bone | ~1.33 x 1025 | |
| mineral | calcium phosphate | all | mineralized tissue | 1.33 x 1025[2] | |
| all | all | all | "standard man" | 1.52 x 1025[1] |
Acknowledgements
The content on this page was first contributed by: Henry A. Hoff.
Initial content for this page in some instances came from Wikipedia.
References
- ↑ 1.0 1.1 CRC Handbook of Chemistry and Physics (88th ed.). Boca Raton, Florida: CRC Press. 2007–2008. p. 7-18. Unknown parameter
|editor-in-chief=ignored (help) - ↑ 2.0 2.1 2.2 2.3 2.4 Schwartz MK. "Phosphate metabolism". McGraw-Hill Encyclopedia of Science & Technology (9th ed.). 13: 343–4.
- ↑ Buono MJ, Kolkhorst FW (2001). "Estimating ATP resynthesis during a marathon run: a method to introduce metabolism" (PDF). Adv Physiol Educ. 25 (2): 70–1.
- ↑ Guigas G, Weiss M (2008). "Sampling the Cell with Anomalous Diffusion—The Discovery of Slowness". Biophys J. 94 (1): 90–4. doi:10.1529/biophysj.107.117044. PMID 17827216. Unknown parameter
|month=ignored (help) - ↑ 5.0 5.1 Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A, Terkeltaub R (2001). "PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification". Am J Pathol. 158 (2): 543–54. PMID 11159191. Unknown parameter
|month=ignored (help) - ↑ 6.0 6.1 6.2 Ho AM, Johnson MD, Kingsley DM (2000). "Role of the mouse ank gene in control of tissue calcification and arthritis". Science. 289 (5477): 265–70. doi:10.1126/science.289.5477.265. PMID 10894769. Unknown parameter
|month=ignored (help) - ↑ 7.0 7.1 7.2 7.3 Ross, Michael H., Gordon I. Kaye, and Wojciech Pawlina, "Histology: a Text and Atlas", 4th ed. (Baltimore: Lippincott Williams & Wilkins, 2002), p. 441.
- ↑ Cate, A. R. Ten, "Oral Histology: Development, Structure, and Function", 5th ed. (Saint Louis: Mosby-Year Book, 1998), p. 1.
- ↑ 9.0 9.1 Cate, A. R. Ten, "Oral Histology: Development, Structure, and Function", 5th ed. (Saint Louis: Mosby-Year Book, 1998), p. 219.
- ↑ 10.0 10.1 10.2 Johnson, Clarke. "Biology of the Human Dentition," 1998. Page accessed on January 24, 2007.
- ↑ Cate, A.R. Ten. Oral Histology: development, structure, and function. 5th ed. 1998. Page 150. ISBN 0-8151-2952-1.
- ↑ Ross, Michael H., Gordon I. Kaye, and Wojciech Pawlina, 2003. Histology: a text and atlas. 4th edition. Page 448. ISBN 0-683-30242-6.
- ↑ Anderson DJ (1949). "Nitrogen in Human Dental Enamel" (PDF). Biochem J. 45 (1): 31–2. PMID 16748584.