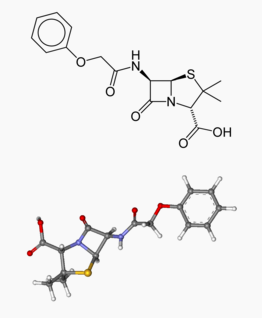
Phenoxymethylpenicillin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Phenoxymethylpenicillin is an antibiotic that is FDA approved for the treatment of streptococcal infections (without bacteremia), pneumococcal infections, Staphylococcal infections – penicillin G-sensitive and fusospirochetosis (Vincent’s gingivitis and pharyngitis). Common adverse reactions include black hairy tongue, diarrhea, epigastric discomfort, nausea and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Penicillin V potassium tablets are indicated in the treatment of mild to moderately severe infections due to penicillin G-sensitive microorganisms. The following infections will usually respond to adequate dosage of penicillin V:
- Streptococcal Infections (without bacteremia)
- Mild-to-moderate infections of the upper respiratory tract, scarlet fever, and mild erysipelas.
- Dosage: 125 to 250 mg (200,000 to 400,000 units) every 6 to 8 hours for 10 days.
- Mild to moderately severe infections of the respiratory tract.
- Dosage: 250 to 500 mg (400,000 to 800,000 units) every 6 hours until the patient has been afebrile for at least 2 days.
- Staphylococcal infections – penicillin G-sensitive
- Mild infections of the skin and soft tissues.
- Dosage: 250 to 500 mg (400,000 to 800,000 units) every 6 to 8 hours.
- Fusospirochetosis (Vincent’s gingivitis and pharyngitis)
- Mild to moderately severe infections of the oropharynx usually respond to therapy with oral penicillin.
- Dosage: 250 to 500 mg (400,000 to 800,000 units) every 6 to 8 hours.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Phenoxymethylpenicillin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Phenoxymethylpenicillin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Streptococcal Infections (without bacteremia)
- Mild-to-moderate infections of the upper respiratory tract, scarlet fever, and mild erysipelas.
- Dosage:
- 12 years and older: 125 to 250 mg (200,000 to 400,000 units) ORALLY every 6 to 8 hours for 10 days
- Less than 12 years of age: 25 to 50 mg/kg/day ORALLY in 3 to 4 divided doses, maximum 3 g/day
- Older than 3 months of age: 50 to 75 mg/kg/day ORALLY in 3 to 4 divided doses
- Mild to moderately severe infections of the respiratory tract.
- Dosage:
- 12 years and older: 250 to 500 mg (400,000 to 800,000 units) ORALLY every 6 hours until patient has been afebrile for at least 2 days
- Less than 12 years of age: 25 to 50 mg/kg/day ORALLY in 3 to 4 divided doses, maximum 3 g/day
Staphylococcal infections – penicillin G-sensitive
- Mild infections of the skin and soft tissues.
- Dosage:
- 12 years and older: 250 to 500 mg (400,000 to 800,000 units) ORALLY every 6 to 8 hours
- Less than 12 years of age: 25 to 50 mg/kg/day ORALLY in 3 to 4 divided doses, maximum 3 g/day
Fusospirochetosis (Vincent’s gingivitis and pharyngitis)
- Mild to moderately severe infections of the oropharynx usually respond to therapy with oral penicillin.
- Dosage:
- 12 years and older: 250 to 500 mg (400,000 to 800,000 units) ORALLY every 6 to 8 hours
- less than 12 years of age: 25 to 50 mg/kg/day ORALLY in 3 to 4 divided doses, maximum 3 g/day
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Phenoxymethylpenicillin in pediatric patients.
Non–Guideline-Supported Use
- Prophylaxis of pneumococcal infectious disease in patients with sickle cell disease and asplenia
Contraindications
A previous hypersensitivity reaction to any penicillin is a contraindication.
Warnings
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (anaphylactic) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE INITIATING THERAPY WITH PENICILLIN V POTASSIUM TABLETS, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS, OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, PENICILLIN V POTASSIUM TABLETS SHOULD BE DISCONTINUED AND APPROPRIATE THERAPY INSTITUTED. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including penicillin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Adverse Reactions
Clinical Trials Experience
Although the incidence of reactions to oral penicillins has been reported with much less frequency than following parenteral therapy, it should be remembered that all degrees of hypersensitivity, including fatal anaphylaxis, have been reported with oral penicillin.
The most common reactions to oral penicillin are nausea, vomiting, epigastric distress, diarrhea, and black hairy tongue. The hypersensitivity reactions reported are skin eruptions (maculopapular to exfoliative dermatitis), urticaria and other serum-sicknesslike reactions, laryngeal edema, and anaphylaxis.
Fever and eosinophilia may frequently be the only reaction observed. Hemolytic anemia, leukopenia, thrombocytopenia, neuropathy, and nephropathy are infrequent reactions and usually associated with high doses of parenteral penicillin.
Postmarketing Experience
There is limited information regarding Phenoxymethylpenicillin Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Phenoxymethylpenicillin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
There is no FDA guidance on usage of Phenoxymethylpenicillin in women who are pregnant.
Pregnancy Category (AUS): A
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Phenoxymethylpenicillin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Phenoxymethylpenicillin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Phenoxymethylpenicillin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Phenoxymethylpenicillin in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Phenoxymethylpenicillin in geriatric settings.
Gender
There is no FDA guidance on the use of Phenoxymethylpenicillin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Phenoxymethylpenicillin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Phenoxymethylpenicillin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Phenoxymethylpenicillin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Phenoxymethylpenicillin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Phenoxymethylpenicillin in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Phenoxymethylpenicillin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Phenoxymethylpenicillin and IV administrations.
Overdosage
There is limited information regarding Phenoxymethylpenicillin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
Penicillin V exerts a bactericidal action against penicillin-sensitive microorganisms during the stage of active multiplication. It acts through the inhibition of biosynthesis of cell-wall mucopeptide. It is not active against the penicillinase-producing bacteria, which include many strains of staphylococci. The drug exerts high in vitro activity against staphylococci (except penicillinase-producing strains), streptococci (groups A, C, G, H, L and M), and pneumococci. Other organisms sensitive in vitro to penicillin V are Corynebacteriumdiphtheriae, Bacillus anthracis, Clostridia, Actinomycesbovis, Streptobacillusmoniliformis, Listeria monocytogenes, Leptospira, and Neisseria gonorrhoeae. Treponemapallidum is extremely sensitive.
Structure

Pharmacodynamics
There is limited information regarding Phenoxymethylpenicillin Pharmacodynamics in the drug label.
Pharmacokinetics
The potassium salt of penicillin V has the distinct advantage over penicillin G in resistance to inactivation by gastric acid. It may be given with meals; however, blood levels are slightly higher when the drug is given on an empty stomach. Average blood levels are two to five times higher than the levels following the same dose of oral penicillin G and also show much less individual variation.
Once absorbed, penicillin V is about 80% bound to serum protein. Tissue levels are highest in the kidneys, with lesser amounts in the liver, skin, and intestines. Small amounts are found in all other body tissues and the cerebrospinal fluid. The drug is excreted as rapidly as it is absorbed in individuals with normal kidney function; however, recovery of the drug from the urine indicates that only about 25% of the dose given is absorbed. In neonates, young infants, and individuals with impaired kidney function, excretion is considerably delayed.
Nonclinical Toxicology
There is limited information regarding Phenoxymethylpenicillin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Phenoxymethylpenicillin Clinical Studies in the drug label.
How Supplied
Phenomethylpenicillin Tablets 250 mg (400,000 units)
- bottles of 100
- bottles of 1000
Phenomethylpenicillin Tablets 500 mg (800,000 units)
- bottles of 100
- bottles of 1000
Storage
Store at 20°-25°C (68°-77°F)
Images
Drug Images
{{#ask: Page Name::Phenoxymethylpenicillin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

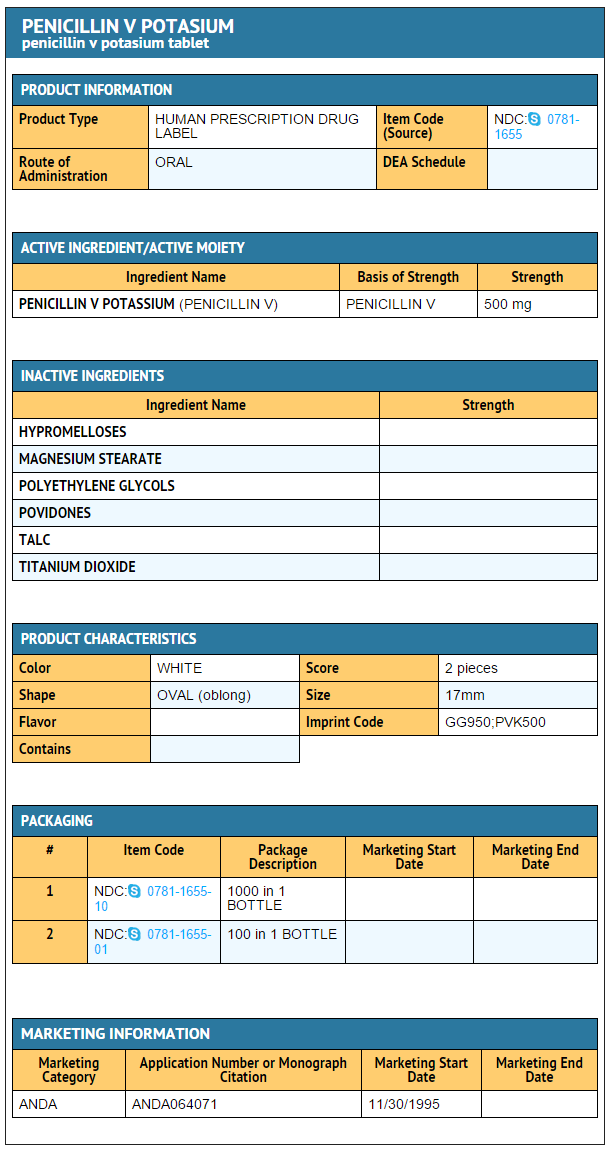
{{#ask: Label Page::Phenoxymethylpenicillin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients should be counseled that antibacterial drugs including penicillin-VK should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When penicillin-VK is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may:
- Decrease the effectiveness of the immediate treatment, and
- Increase the likelihood that bacteria will develop resistance and will not be treatable by penicillin-VK or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Precautions with Alcohol
Alcohol-Phenoxymethylpenicillin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Veetids [1]
- Truxcillin VK
Look-Alike Drug Names
There is limited information regarding Phenoxymethylpenicillin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Phenoxymethylpenicillin |Label Name=Phenoxymethylpenicillin 250 mg.png
}}
{{#subobject:
|Label Page=Phenoxymethylpenicillin |Label Name=Phenoxymethylpenicillin 500 mg.png
}}