Tafenoquine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tafenoquine is an antimalarial that is FDA approved for the radical cure (prevention of relapse) of Plasmodium vivax malaria in patients aged 16 years and older who are receiving appropriate antimalarial therapy for acute P. vivax infection. Common adverse reactions include dizziness, nausea, vomiting, headache, and decreased hemoglobin.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Tafenoquine is indicated for the radical cure (prevention of relapse) of Plasmodium vivax malaria in patients aged 16 years and older who are receiving appropriate antimalarial therapy for acute P. vivax infection.
Limitation of Use
Dosage
- The recommended dose of tafenoquine in patients aged 16 years and older is a single dose of 300 mg administered as two 150-mg tafenoquine tablets taken together.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding tafenoquine Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding tafenoquine Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of tafenoquine in pediatric patients younger than 16 years have not been established.
- For patients of ages 16 and older, see Adult Indication and Dosage.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding tafenoquine Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding tafenoquine Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Tafenoquine is contraindicated in:
- patients with G6PD deficiency or unknown G6PD status due to the risk of hemolytic anemia
- breastfeeding by a lactating woman when the infant is found to be G6PD deficient or if the G6PD status of the infant is unknown
- patients with known hypersensitivity to tafenoquine, other 8-aminoquinolines, or any component of tafenoquine
Warnings
Hemolytic Anemia
- Due to the risk of hemolytic anemia in patients with G6PD deficiency, G6PD testing must be performed before prescribing tafenoquine. Due to the limitations of G6PD tests, physicians need to be aware of residual risk of hemolysis and adequate medical support and follow-up to manage hemolytic risk should be available. Treatment with tafenoquine is contraindicated in patients with G6PD deficiency or unknown G6PD status. Patients were excluded from clinical trials of tafenoquine if they had a G6PD enzyme activity level <70% of the site median value for G6PD normal activity. In clinical trials, declines in hemoglobin levels were reported in some G6PD-normal patients. Monitor patients for clinical signs or symptoms of hemolysis. Advise patients to seek medical attention if signs of hemolysis occur.
G6PD Deficiency in Pregnancy or Lactation
Potential Harm to the Fetus
- The use of tafenoquine during pregnancy may cause hemolytic anemia in a G6PD-deficient fetus. Even if a pregnant woman has normal levels of G6PD, the fetus could be G6PD deficient. Advise females of reproductive potential that treatment with tafenoquine during pregnancy is not recommended and to avoid pregnancy or use effective contraception for 3 months after the dose of tafenoquine.
Potential Harm to the Breastfeeding Infant
- A G6PD-deficient infant may be at risk for hemolytic anemia from exposure to tafenoquine through breast milk. Infant G6PD status should be checked before breastfeeding begins. Tafenoquine is contraindicated in breastfeeding women when the infant is found to be G6PD-deficient or the G6PD status of the infant is unknown. Advise the woman with a G6PD-deficient infant or if the G6PD status of the infant is unknown not to breastfeed for 3 months after the dose of tafenoquine.
Methemoglobinemia
- Asymptomatic elevations in methemoglobin have been observed in the clinical trials of tafenoquine. Institute appropriate therapy if signs or symptoms of methemoglobinemia occur. Carefully monitor individuals with nicotinamide adenine dinucleotide (NADH)-dependent methemoglobin reductase deficiency. Advise patients to seek medical attention if signs of methemoglobinemia occur.
Psychiatric Effects
- Psychiatric adverse reactions including anxiety (<1%), abnormal dreams (<1%), and insomnia (3%) have been reported in clinical trials of tafenoquine. Two cases of depression and 2 cases of psychosis have occurred primarily in patients with a history of psychiatric disorders following receipt of single doses of tafenoquine that were higher than the approved 300-mg dose (350 mg to 600 mg). Safety and effectiveness of tafenoquine have not been established at doses or regimens other than the approved regimen; use of tafenoquine at doses or regimens other than a 300-mg single dose is not approved by FDA.
- The benefit of treatment with tafenoquine must be weighed against the potential risk for psychiatric adverse reactions in patients with a history of psychiatric illness. Due to the long half-life of tafenoquine (approximately 15 days), signs or symptoms of psychiatric adverse reactions that may occur could be delayed in onset and/or duration.
Hypersensitivity Reactions
- Serious hypersensitivity reactions (e.g., angioedema, urticaria) have been observed with administration of tafenoquine. Institute appropriate therapy if hypersensitivity reactions occur. Do not re-administer tafenoquine. Tafenoquine is contraindicated in patients who develop hypersensitivity to tafenoquine or any component of tafenoquine or other 8-aminoquinolines.
- Due to the long half-life of tafenoquine (approximately 15 days), signs or symptoms of hypersensitivity adverse reactions that may occur could be delayed in onset and/or duration. Advise patients to seek medical attention if signs of hypersensitivity occur.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety data described below reflect exposure to 4,129 subjects, of whom 810 received a 300-mg single dose of tafenoquine. Tafenoquine was evaluated in patients with P. vivax malaria (n = 483) in 3 randomized, double-blind trials including a placebo-controlled trial comparing tafenoquine plus chloroquine (n = 260) with chloroquine alone (Trial 1), a placebo-controlled dose-ranging trial (Trial 2) (n = 57), and a hematologic safety trial (Trial 3, NCT02216123) (n = 166).
- In Trial 1, in patients with P. vivax malaria, the most common adverse reactions reported in ≥5% of patients treated with tafenoquine are listed in TABLE 1. Patients included in the trial had a mean age of 35 (range: 16 to 79 years), were 75% male and from the following regions: 70% Latin America (Brazil and Peru), 19% Southeast (SE) Asia (Thailand, Cambodia, and the Philippines), and 11% Africa (Ethiopia).
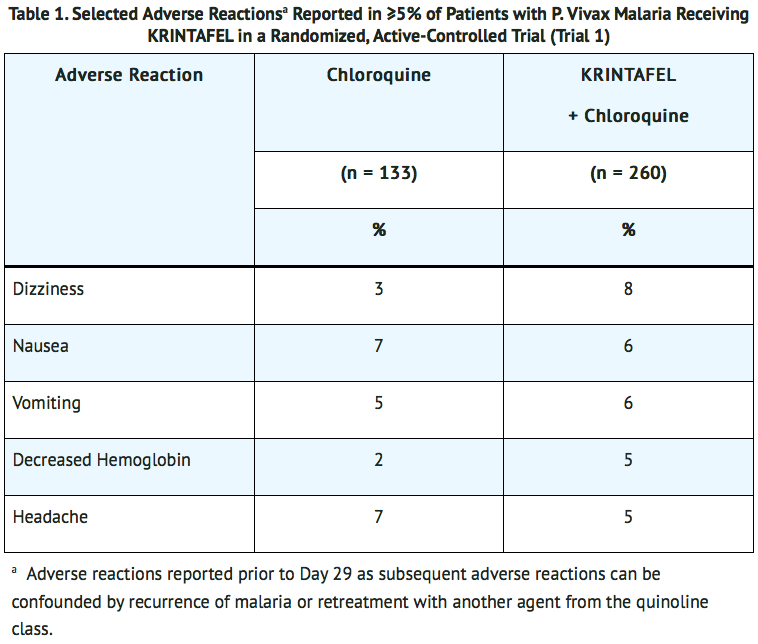
Other Adverse Reactions Reported with tafenoquine
- Clinically significant adverse reactions with tafenoquine 300-mg single dose in clinical trials (n = 810) in ≤3% of subjects are listed below:
- Psychiatric Disorders: Anxiety, insomnia, abnormal dreams.
- Nervous System Disorders: Somnolence.
- Laboratory Investigations: Increased blood creatinine, increased blood methemoglobin, increased alanine aminotransferase.
- Immune System Disorders: Hypersensitivity reactions (e.g., angioedema, urticaria).
- Eye Disorders: Vortex keratopathy, photophobia.
Postmarketing Experience
There is limited information regarding Tafenoquine Postmarketing Experience in the drug label.
Drug Interactions
Effect of Tafenoquine on Organic Cation Transporter2 (OCT2) and Multidrug and Toxin Extrusion (MATE) Substrates
- The effect of coadministration of tafenoquine on the pharmacokinetics of OCT2 and MATE substrates in humans is unknown. However, in vitro, observations suggest the potential for increased concentrations of these substrates which may increase the risk of toxicity of these drugs.
- Avoid coadministration of tafenoquine with OCT2 and MATE substrates (e.g., dofetilide, metformin). If coadministration cannot be avoided, monitor for drug-related toxicities and consider dosage reduction if needed based on approved product labeling of the coadministered drug.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- The use of tafenoquine during pregnancy may cause hemolytic anemia in a fetus who is G6PD deficient. Treatment with tafenoquine during pregnancy is not recommended. Available data with use of tafenoquine in pregnant women are insufficient to establish a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal studies, there were increased abortions, with and without maternal toxicity, when tafenoquine was given orally to pregnant rabbits at and above doses equivalent to about 0.4 times the clinical exposure based on body surface area comparisons. No fetotoxicity was observed at doses equivalent to the clinical exposure (based on body surface area comparisons) in a similar study in rats.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
- Disease-Associated Maternal and/or Embryo/Fetal Risk: Malaria during pregnancy increases the risk for adverse pregnancy outcomes, including maternal anemia, prematurity, spontaneous abortion, and stillbirth.
Data
- Animal Data: Tafenoquine resulted in dose-related abortions when given orally to pregnant rabbits during organogenesis (Gestation Days 6 to 18) at doses of 7 mg/kg (about 0.4 times the clinical exposure based on body surface area comparisons) and above. Doses higher than 7 mg/kg were also associated with maternal toxicity (mortality and reduced body weight gain). In a similar study in rats, doses of 3, 10, or 30 mg/kg/day resulted in maternal toxicity (enlarged spleen, reduced body weight, and reduced food intake) but no fetotoxicity at the high dose (equivalent to the clinical exposure based on body surface area comparisons). There was no evidence of malformations in either species. In a pre- and postnatal development study in rats, tafenoquine administered throughout pregnancy and lactation produced maternal toxicity and a reversible decrease in offspring body weight gain and decrease in motor activity at 18 mg/kg/day, which is equivalent to about 0.6 times the clinical dose based on body surface area comparisons.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tafenoquine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tafenoquine during labor and delivery.
Nursing Mothers
Risk Summary
- A breastfed infant with G6PD deficiency is at risk for hemolytic anemia from exposure to tafenoquine. Infant G6PD status should be checked before breastfeeding begins. Tafenoquine is contraindicated in breastfeeding women when the infant is found to be G6PD deficient or the G6PD status of the infant is unknown.
- There is no information regarding the presence of tafenoquine in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. In a breastfed infant with normal G6PD, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for tafenoquine and any potential effects on the breastfed infant from tafenoquine or from the underlying maternal condition.
Clinical Considerations
- Check the infant’s G6PD status before maternal breastfeeding commences. If an infant is G6PD deficient, exposure to tafenoquine during breastfeeding may result in hemolytic anemia in the infant; therefore, advise the woman with an infant who has G6PD deficiency or whose G6PD status is unknown, not to breastfeed for 3 months after the dose of tafenoquine.
Pediatric Use
- The safety and effectiveness of tafenoquine have been established in pediatric patients aged 16 years and older. Use of tafenoquine in these pediatric patients is supported by evidence from adequate and well-controlled studies of tafenoquine.
- Safety and effectiveness of tafenoquine in pediatric patients younger than 16 years have not been established.
Geriatic Use
- Clinical trials of tafenoquine did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Tafenoquine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tafenoquine with respect to specific racial populations.
Renal Impairment
- The pharmacokinetics of tafenoquine have not been studied in patients with renal impairment. If tafenoquine is administered to such patients, monitoring for adverse reactions associated with tafenoquine is needed.
Hepatic Impairment
- The pharmacokinetics of tafenoquine have not been studied in patients with hepatic impairment. If tafenoquine is administered to such patients, monitoring for adverse reactions associated with tafenoquine is needed.
Females of Reproductive Potential and Males
Pregnancy Testing
- Verify the pregnancy status in females of reproductive potential prior to initiating treatment with tafenoquine.
Contraception
- Tafenoquine may cause hemolytic anemia in a G6PD-deficient fetus. Advise females of reproductive potential that treatment with tafenoquine during pregnancy is not recommended and to avoid pregnancy or use effective contraception for 3 months after the dose of tafenoquine.
Immunocompromised Patients
There is no FDA guidance one the use of Tafenoquine in patients who are immunocompromised.
Administration and Monitoring
Administration
Tests to be Performed Prior to Treatment with Tafenoquine
- All patients must be tested for glucose-6-phosphate dehydrogenase (G6PD) deficiency prior to prescribing tafenoquine.
- Pregnancy testing is recommended for females of reproductive potential prior to initiating treatment with tafenoquine.
Recommended Dosage and Administration
- The recommended dose of tafenoquine in patients aged 16 years and older is a single dose of 300 mg administered as two 150-mg tablets taken together. Coadminister tafenoquine on the first or second day of the appropriate antimalarial therapy (e.g. chloroquine) for acute P. vivax malaria.
- Administer tafenoquine with food to increase systemic absorption.
- Swallow tablets whole. Do not break, crush, or chew the tablets.
- In the event of vomiting within 1 hour after dosing, a repeat dose should be given. Re-dosing should not be attempted more than once.
Monitoring
There is limited information regarding Tafenoquine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tafenoquine and IV administrations.
Overdosage
- Hemoglobin decline and methemoglobinemia may be encountered in an overdose with tafenoquine. Treatment of overdosage consists of institution of appropriate symptomatic and/or supportive therapy.
Pharmacology

| |
Tafenoquine
| |
| Systematic (IUPAC) name | |
| N-[2,6-Dimethoxy-4-methyl-5-[3-(trifluoromethyl)phenoxy]quinolin-8-yl]pentane-1,4-diamine | |
| Identifiers | |
| CAS number | |
| ATC code | P01 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 463.493 g/mol |
| SMILES | & |
| Synonyms | Etaquine,[1] WR 238605,[1] SB-252263 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Tafenoquine is an 8-aminoquinoline antimalarial drug.
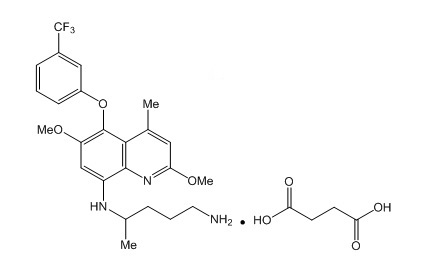
Structure
- The molecular formula of tafenoquine succinate is C24H28F3N3O3 • C4H6O4, and its molecular mass is 581.6 as the succinate salt (463.5 as free base). The structural formula is shown below.
Pharmacodynamics
Cardiac Electrophysiology
- The effect of tafenoquine on the QTc interval was evaluated in a Phase 1 randomized, single-blind, placebo- and positive-controlled, parallel-group thorough QTc study in 260 healthy adult subjects. At a cumulative dose of 1,200 mg (400 mg/day for 3 days; 4 times the maximum recommended dose), tafenoquine did not prolong the QTc interval to any clinically relevant extent.
Exposure-Response Relationships
- A saturable relationship between tafenoquine exposure (AUC) and clinical response (recurrence-free rate at 6 months) was identified. Tafenoquine exposures achieved with doses of 300 mg and higher are on the plateau of the exposure-response curve. Use of tafenoquine at doses or regimens other than a 300-mg single dose is not approved by the FDA.
Pharmacokinetics
Absorption
- Maximum plasma concentrations were generally observed 12 to 15 hours following oral administration.
- Food Effect: Plasma tafenoquine AUC increased by 41% and Cmax increased by 31% when administered as an investigational capsule formulation with a high-calorie, high-fat meal (approximately 1,000 calories with 15% protein, 25% carbohydrate, and 60% fat) compared with the fasted state.
Distribution
- Protein binding of tafenoquine is >99.5%. The apparent oral volume of distribution is ~1,600 L. Following single- and multiple-oral-dose administration, tafenoquine whole blood concentrations were on average 67% higher than corresponding plasma values.
Elimination
- The apparent oral clearance of tafenoquine is approximately 3 L/h. The average terminal half-life is approximately 15 days.
- Metabolism: Tafenoquine undergoes slow metabolism. Unchanged tafenoquine represented the only notable drug-related component in human plasma after a single oral dose of tafenoquine.
- Excretion: The full excretion profile of tafenoquine in humans is unknown. Over a 6-day collection period, renal elimination of unchanged tafenoquine was low.
Specific Populations
- Pharmacokinetics of tafenoquine were not significantly impacted by age, sex, ethnicity, and body weight. The effect of renal or hepatic impairment on tafenoquine pharmacokinetics is unknown.
Drug Interaction Studies
- Clinical Studies: No clinically significant effects on tafenoquine pharmacokinetics were observed following coadministration with chloroquine, dihydroartemisinin-piperaquine, or artemether-lumefantrine in healthy subjects.
- No clinically significant effects on the pharmacokinetics of dihydroartemisinin, piperaquine, artemether, lumefantrine, or substrates of cytochrome P450 isoenzymes (CYP)1A2 (caffeine), CYP2D6 (desipramine), CYP2C8 (chloroquine), CYP2C9 (flurbiprofen), or CYP3A4 (midazolam, chloroquine) were observed following coadministration of tafenoquine in healthy subjects.
- In Vitro Studies Where Drug Interaction Potential Was Not Further Evaluated Clinically: Tafenoquine inhibited metformin transport via human OCT2, MATE-1, and MATE2-K transporters. Clinical drug interaction studies with tafenoquine and OCT2 and MATE substrates have not been conducted.
- The effect of tafenoquine on substrates of P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and organic anion transporting polypeptides 1B1/1B3 (OATP1B1/OATP1B3) is unknown.
Microbiology Mechanism of Action
- Tafenoquine, an 8-aminoquinoline antimalarial, is active against the liver stages including the hypnozoite (dormant stage) of P. vivax. In addition to its effect on the parasite, tafenoquine causes red blood cell shrinkage in vitro. The molecular target of tafenoquine is not known.
Antimicrobial Activity
- Tafenoquine is active against pre-erythrocytic (liver) and erythrocytic (asexual) forms as well as gametocytes of P. vivax. The activity of tafenoquine against the pre-erythrocytic liver stages of the parasite prevents the development of the erythrocytic forms of the parasite, which are responsible for relapses in P. vivax malaria.
Resistance
- A potential for development of resistance of Plasmodium species to tafenoquine was not evaluated.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Two-year oral carcinogenicity studies were conducted in rats and mice. Renal cell adenomas and carcinomas were increased in male rats at doses of 1 mg/kg/day and above (3 times the clinical exposure based on AUC comparisons). Tafenoquine was not carcinogenic in mice. Given the single-dose administration of tafenoquine, these findings may not represent a carcinogenicity risk to humans.
Mutagenesis
- Tafenoquine did not cause mutations or chromosomal damage in 2 definitive in vitro tests (bacterial mutation assay and mouse lymphoma L5178Y cell assay) or in an in vivo oral rat micronucleus test.
Impairment of Fertility
- In a rat fertility study, tafenoquine was given orally at 1.5, 5, and 15 mg/kg/day (up to about 0.5 times the human dose based on body surface area comparisons) to males for at least 67 days, including 29 days prior to mating, and to females from 15 days prior to mating through early pregnancy. Tafenoquine resulted in reduced number of viable fetuses, implantation sites, and corpora lutea at 15 mg/kg in the presence of maternal toxicity (mortality, piloerection, rough coat, and reduced body weight).
Clinical Studies
- Trial 1 (NCT01376167) was a double-blind, controlled clinical trial of 522 adults positive for P. vivax across 3 regions (Asia, Africa, and Latin America). All patients received chloroquine phosphate (600-mg free base on Days 1 and 2 with 300-mg free base on Day 3) to treat the acute infection in addition to either a one-time dose of tafenoquine (two 150-mg tablets) on Day 1 or Day 2 (n = 260), an active control (n = 129), or placebo (n = 133) in a 2:1:1 fashion. Patients included in the trial had a mean age of 35 (range: 16 to 79 years), were 75% male and from the following regions: 70% Latin America (Brazil and Peru), 19% SE Asia (Thailand, Cambodia, and the Philippines), and 11% Africa (Ethiopia).
- Patients were considered recurrence-free at 6 months if they demonstrated initial parasite clearance, took no anti-malarial medications, and were confirmed parasite-free at the 6-month final assessment (i.e., absence of relapse or new infection).
- Due to the risk of hemolytic anemia, patients were excluded from the trial if they had a G6PD enzyme activity level <70% of the site median value for G6PD normals (8.2 IU/gHb). In this trial, the minimum G6PD enzyme level of any subject was 5.4 IU/gHb. Patients with severe malaria were excluded from the trial.
- The recurrence-free efficacy rates at 6 months among the tafenoquine and placebo groups are presented in TABLE 2. The risk of recurrence for tafenoquine plus chloroquine was reduced by 76% compared with placebo plus chloroquine.
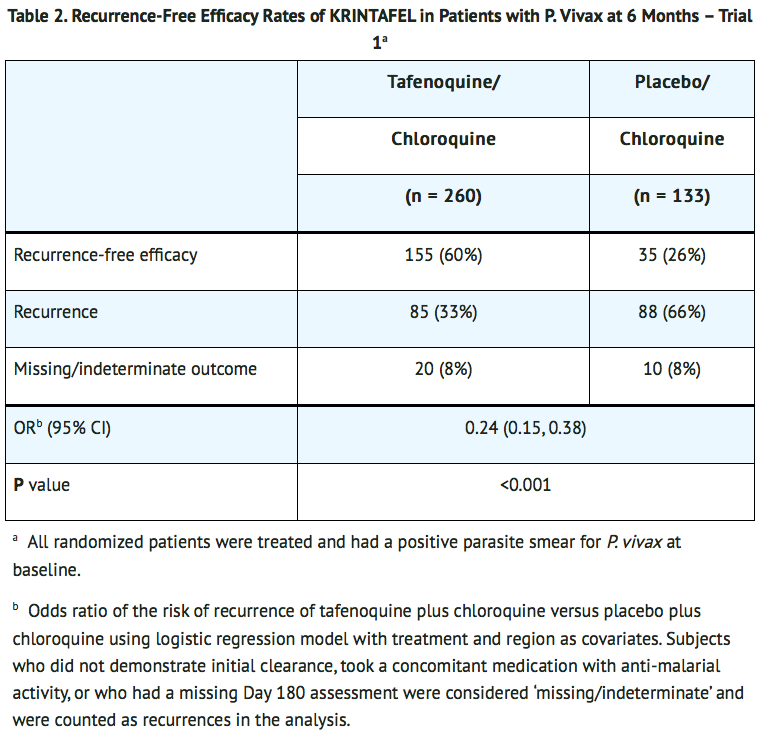
- In Trial 2 (NCT01376167), a dose-ranging trial with a study design similar to Trial 1, 57 and 54 subjects were randomized to tafenoquine 300-mg single dose plus chloroquine (same dose as in Trial 1) and placebo plus chloroquine groups, respectively. Tafenoquine plus chloroquine demonstrated a statistically significantly higher rate of recurrence-free efficacy at 6 months compared with the placebo plus chloroquine control group (84% versus 39%, with a difference of 45% and 95% CI [29%, 61%]).
How Supplied
- Tafenoquine tablets contain 150 mg of tafenoquine (equivalent to 188.2 mg tafenoquine succinate) and are pink, film‑coated, capsule-shaped, and debossed with ‘GS J11’ on one side. Tafenoquine is supplied as follows:
- Bottle of 30 tablets with child-resistant closure (NDC 0173-0889-13). Bottles contain a desiccant. Once opened, use within 3 months.
- Unit Dose Pack of 2 tablets in a bottle with child-resistant closure (NDC 0173-0889-39). Bottles contain a desiccant.
Storage
- Store at 20°C to 25°C (68°F to 77°F). Temperature excursions are permitted to 15°C to 30°C (59°F to 86°F).
- Store in the original package to protect from moisture. Keep the bottle tightly closed and do not remove the desiccant.
Images
Drug Images
{{#ask: Page Name::Tafenoquine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Tafenoquine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
G6PD Testing and Hemolytic Anemia
- Inform patients of the need for testing for G6PD deficiency before starting tafenoquine. Advise patients of the symptoms of hemolytic anemia and instruct them to seek medical advice promptly if such symptoms occur. Patients should contact their healthcare provider if they develop dark lips or urine as these may be signs of hemolysis or methemoglobinemia.
Important Administration Instructions
- Advise patients to take tafenoquine with food to increase absorption.
- Advise patients to swallow the tablet whole and not to break, crush, or chew it.
Potential Harm to the Fetus
- Advise females of reproductive potential of the potential risk of tafenoquine to a fetus and to inform their healthcare provider of a known or suspected pregnancy.
- Advise females of reproductive potential to avoid pregnancy or use effective contraception for 3 months after the dose of tafenoquine.
Lactation
- Advise women with a G6PD-deficient infant, or if they do not know the G6PD status of their infant, not to breastfeed for 3 months after the dose of tafenoquine.
Methemoglobinemia
- Inform patients that methemoglobinemia has occurred with tafenoquine. Advise patients of the symptoms of methemoglobinemia and instruct them to seek medical advice promptly if such symptoms occur.
Psychiatric Symptoms
- Advise patients with a history of psychiatric illness regarding the potential for new or worsening psychiatric symptoms with tafenoquine and instruct them to seek medical advice promptly if such symptoms occur.
Hypersensitivity Reactions
- Inform patients that hypersensitivity reactions have occurred with tafenoquine. Advise patients of the symptoms of hypersensitivity reactions and instruct them to seek medical advice promptly if such symptoms occur.
Patient Package Insert

Precautions with Alcohol
Alcohol-Tafenoquine interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Tafenoquine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.