Amitriptyline
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Suicidality and Antidepressant Drugs
See full prescribing information for complete Boxed Warning.
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of amitriptyline hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Amitriptyline hydrochloride is not approved for use in pediatric patients.
|
Overview
Amitriptyline is a Tricyclic antidepressant that is FDA approved for the {{{indicationType}}} of depression. There is a Black Box Warning for this drug as shown here. Common adverse reactions include weight gain, constipation, xerostomia, dizziness, headache, somnolence, blurred vision.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Depression
- Outpatients: 75 mg ORALLY (divided into 1 to 3 doses per day); may increase up to a max of 150 mg/day
- Inpatients: 100 mg ORALLY (divided into 1 to 3 doses per day); may increase up to a max of 300 mg/day
- Maintenance: 50 to 100 mg ORALLY at bedtime
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Amitriptyline in adult patients.
Non–Guideline-Supported Use
Headache
Treatment and ProphylaxisView additional informationIrritable bowel syndrome
Pain
Polyneuropathy
Postherpetic neuralgia
Treatment and Prophylaxis- There is limited information about Off-Label Non–Guideline-Supported Use of Amitriptyline in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Amitriptyline FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Amitriptyline in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Amitriptyline in pediatric patients.
Contraindications
- Hypersensitivity to tricyclic antidepressants or to any of its recipients
- History of myocardial infarction
- History of arrhythmias, and heart block to any degree
- Congestive heart failure
- Coronary artery insufficiency
- Mania
- Severe liver disease
- Children under 7 years
- Breast feeding
- Patients who are taking monoamine oxidase inhibitors (MAOIs) or have taken them within the last 14 days.
Warnings
|
Suicidality and Antidepressant Drugs
See full prescribing information for complete Boxed Warning.
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of amitriptyline hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Amitriptyline hydrochloride is not approved for use in pediatric patients.
|
Clinical Worsening and Suicide Risk
- Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
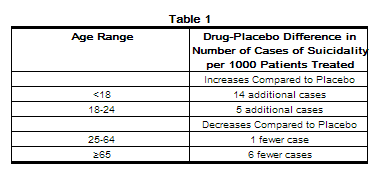
- The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
- No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
- It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
- All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
- The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
- Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
- Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for amitriptyline hydrochloride should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder
- A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that amitriptyline hydrochloride is not approved for use in treating bipolar depression.
- Amitriptyline hydrochloride may block the antihypertensive action of guanethidine, or similarly acting compounds.
- It should be used with caution in patients with a history of seizures and, because of its atropine-like action, in patients with a history of urinary retention, angle-closure glaucoma or increased intraocular pressure. In patients with angle-closure glaucoma, even average doses may precipitate an attack.
- Patients with cardiovascular disorders should be watched closely. Tricyclic antidepressant drugs, including amitriptyline hydrochloride, particularly when given in high doses, have been reported to produce arrhythmias, sinus tachycardia, and prolongation of the conduction time. Myocardial infarction and stroke have been reported with drugs of this class.
- Close supervision is required when amitriptyline hydrochloride is given to hyperthyroid patients or those receiving thyroid medication.
- Amitriptyline may enhance the response to alcohol and the effects of barbiturates and other CNS depressants. In patients who may use alcohol excessively, it should be borne in mind that the potentiation may increase the danger inherent in any suicide attempt or overdosage. Delirium has been reported with concurrent administration of amitriptyline and disulfiram.
PRECAUTIONS
- Schizophrenic patients may develop increased symptoms of psychosis; patients with paranoid symptomatology may have an exaggeration of such symptoms. Depressed patients, particularly those with known manic-depressive illness, may experience a shift to mania or hypomania. In these circumstances the dose of amitriptyline may be reduced or a major tranquilizer such as perphenazine may be administered concurrently.
- The possibility of suicide in depressed patients remains until significant remission occurs. Potentially suicidal patients should not have access to large quantities of this drug. Prescriptions should be written for the smallest amount feasible.
- Concurrent administration of amitriptyline hydrochloride and electroshock therapy may increase the hazards associated with such therapy. Such treatment should be limited to patients for whom it is essential.
- When possible, the drug should be discontinued several days before elective surgery.
- Both elevation and lowering of blood sugar levels have been reported.
- Amitriptyline hydrochloride should be used with caution in patients with impaired liver function.
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)Coma; seizures; hallucinations; delusions; confusional states; disorientation; incoordination; ataxia; tremors; peripheral neuropathy; numbness, tingling and paresthesias of the extremities; extrapyramidal symptoms including abnormal involuntary movements and tardive dyskinesia; dysarthria; disturbed concentration; excitement; anxiety; insomnia; restlessness; nightmares; drowsiness; dizziness; weakness; fatigue; headache; syndrome of inappropriate ADH (antidiuretic hormone) secretion; tinnitus; alteration in EEG patterns.
Cardiovascular
- Myocardial infarction; stroke; nonspecific ECG changes and changes in AV conduction; heart block; arrhythmias; hypotension, particularly orthostatic hypotension; syncope; hypertension; tachycardia; palpitation.
Gastrointestinal
- Rarely hepatitis (including altered liver function and jaundice); nausea; epigastric distress; vomiting; anorexia; stomatitis; peculiar taste; diarrhea; parotid swelling; black tongue.
Hypersensitive Reactions
- Skin rash; urticaria; photosensitization; edema of face and tongue.
Anticholinergic
- Paralytic ileus, hyperpyrexia; urinary retention, dilatation of the urinary tract; constipation; blurred vision, disturbance of accommodation, increased ocular pressure, mydriasis; dry mouth.
Hematologic
- Bone marrow depression including agranulocytosis, leukopenia, thrombocytopenia; purpura; eosinophilia.
Endocrine
- Testicular swelling and gynecomastia in the male; breast enlargement and galactorrhea in the female; increased or decreased libido; impotence; elevation and lowering of blood sugar levels.
Miscellaneous
- Alopecia; edema; weight gain or loss; urinary frequency; increased perspiration.
Postmarketing Experience
There is limited information regarding Amitriptyline Postmarketing Experience in the drug label.
Drug Interactions
- CYP2D6 inhibitors and substrates such as fluoxetine
- An increase in plasma concentrations of the drug to be seen.
- It can reduce the antihypertensive effects of this drug.
- Anticholinergic agents such as benztropine, hyoscine (scopolamine) and atropine.
- May exacerbate each other's anticholinergic effects, causing paralytic ileus and tachycardia.
- Exacerbate the sedative, anticholinergic, epileptogenic and pyrexic (fever-promoting) effects.
- Increases the risk of neuroleptic malignant syndrome
- Interfere with hepatic metabolism of amitriptyline, increasing steady-state concentrations of the drug.
- The potential for the development of delirium
- May increase the risks associated with this treatment
- Antithyroid medications
- May increase the risk of agranulocytosis
- Thyroid hormones
- May increase adverse effects such as CNS stimulation and arrhythmias.
- Analgesics, such as tramadol
- May increase in seizure risk.
- Medications that are subject to gastric inactivation (e.g. levodopa)
- Amitriptyline delays gastric emptying and reduce intestinal motility
- Medications that may be subject to increased absorption given more time in the small intestine (e.g. anticoagulants)
- Serotoninergic agents such as the SSRIs and triptans
- Risk of serotonin syndrome.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Teratogenic effects were not observed in mice, rats, or rabbits when amitriptyline was given orally at doses of 2 to 40 mg/kg/day (up to 13 times the maximum recommended human dose1). Studies in literature have shown amitriptyline to be teratogenic in mice and hamsters when given by various routes of administration at doses of 28 to 100 mg/kg/day (9 to 33 times the maximum recommended human dose), producing multiple malformations. Another study in the rat reported that an oral dose of 25 mg/kg/day (8 times the maximum recommended human dose) produced delays in ossification of fetal vertebral bodies without other signs of embryotoxicity. In rabbits, an oral dose of 60 mg/kg/day (20 times the maximum recommended human dose) was reported to cause incomplete ossification of cranial bones.
Amitriptyline has been shown to cross the placenta. Although a causal relationship has not been established, there have been a few reports of adverse events, including CNS effects, limb deformities, or developmental delay, in infants whose mothers had taken amitriptyline during pregnancy.
There are no adequate and well-controlled studies in pregnant women. Amitriptyline hydrochloride should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Based on a maximum recommended amitriptyline dose of 150 mg/day or 3 mg/kg/day for a 50 kg patient.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Amitriptyline in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Amitriptyline during labor and delivery.
Nursing Mothers
Amitriptyline is excreted into breast milk. In one report in which a patient received amitriptyline 100 mg/day while nursing her infant, levels of 83 to 141 ng/mL were detected in the mother’s serum. Levels of 135 to 151 ng/mL were found in the breast milk, but no trace of the drug could be detected in the infant’s serum.
Because of the potential for serious adverse reactions in nursing infants from amitriptyline, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
In view of the lack of experience with the use of this drug in pediatric patients, it is not recommended at the present time for patients under 12 years of age.
Geriatic Use
Clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic function, concomitant disease and other drug therapy in elderly patients.
Geriatric patients are particularly sensitive to the anticholinergic side effects of tricyclic antidepressants including amitriptyline hydrochloride. Peripheral anticholinergic effects include tachycardia, urinary retention, constipation, dry mouth, blurred vision, and exacerbation of narrow-angle glaucoma. Central nervous system anticholinergic effects include cognitive impairment, psychomotor slowing, confusion, sedation, and delirium. Elderly patients taking amitriptyline hydrochloride may be at increased risk for falls. Elderly patients should be started on low doses of amitriptyline hydrochloride and observed closely
Gender
There is no FDA guidance on the use of Amitriptyline with respect to specific gender populations.
Race
There is no FDA guidance on the use of Amitriptyline with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Amitriptyline in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Amitriptyline in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Amitriptyline in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Amitriptyline in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral Dosage
Dosage should be initiated at a low level and increased gradually, noting carefully the clinical response and any evidence of intolerance.
Initial Dosage for Adults
For outpatients, 75 mg of amitriptyline HCl a day in divided doses is usually satisfactory. If necessary, this may be increased to a total of 150 mg per day. Increases are made preferably in the late afternoon and/or bedtime doses. A sedative effect may be apparent before the antidepressant effect is noted, but an adequate therapeutic effect may take as long as 30 days to develop.
An alternate method of initiating therapy in outpatients is to begin with 50 to 100 mg amitriptyline HCl at bedtime. This may be increased by 25 or 50 mg as necessary in the bedtime dose to a total of 150 mg per day.
Hospitalized patients may require 100 mg a day initially. This can be increased gradually to 200 mg a day if necessary. A small number of hospitalized patients may need as much as 300 mg a day.
Adolescent and Elderly Patients
In general, lower dosages are recommended for these patients. Ten mg 3 times a day with 20 mg at bedtime may be satisfactory in adolescent and elderly patients who do not tolerate higher dosages.
Maintenance
The usual maintenance dosage of amitriptyline HCl is 50 to 100 mg per day. In some patients, 40 mg per day is sufficient. For maintenance therapy, the total daily dosage may be given in a single dose, preferably at bedtime. When satisfactory improvement has been reached, dosage should be reduced to the lowest amount that will maintain relief of symptoms. It is appropriate to continue maintenance therapy 3 months or longer to lessen the possibility of relapse.
Usage in Pediatric Patients
In view of the lack of experience with the use of this drug in pediatric patients, it is not recommended at the present time for patients under 12 years of age.
Monitoring
Plasma Levels
Because of the wide variation in the absorption and distribution of tricyclic antidepressants in body fluids, it is difficult to directly correlate plasma levels and therapeutic effect. However, determination of plasma levels may be useful in identifying patients who appear to have toxic effects and may have excessively high levels, or those in whom lack of absorption or noncompliance is suspected. Because of increased intestinal transit time and decreased hepatic metabolism in elderly patients, plasma levels are generally higher for a given oral dose of amitriptyline hydrochloride than in younger patients. Elderly patients should be monitored carefully and quantitative serum levels obtained as clinically appropriate. Adjustments in dosage should be made according to the patient’s clinical response and not on the basis of plasma levels.
IV Compatibility
There is limited information regarding the compatibility of Amitriptyline and IV administrations.
Overdosage
Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose, therefore, hospital monitoring is required as soon as possible.
Manifestations
Critical manifestations of overdose include: cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression, including coma. Changes in the electrocardiogram particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity. In addition, a rightward axis shift in the terminal QRS complex together with a prolonged QT interval and sinus tachycardia are specific and sensitive indicators of first generation tricyclic overdose. The absence of these findings is not exclusionary. Prolonged PR interval, ST-T wave changes, ventricular tachycardia and fibrillation may also occur.
Other signs of overdose may include: impaired myocardial contractility, confusion, disturbed concentration, transient visual hallucinations, dilated pupils, disorders of ocular motility, agitation, hyperactive reflexes polyradiculoneuropathy, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, or any of the symptoms listed under ADVERSE REACTIONS.
Management
General
Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient’s airway, establish an intravenous line and initiate gastric decontamination. A minimum of six hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during the period extended monitoring is required. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
Gastrointestinal Decontamination
All patients suspected of tricyclic antidepressant overdose should receive gastrointestinal decontamination. This should include, large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. EMESIS IS CONTRAINDICATED.
Cardiovascular
A maximal limb-lead QRS duration of ≥0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH >7.60 or a pCO2 <20 mm Hg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium or phenytoin. Type 1 A and 1 C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic antidepressant poisoning.
CNS
In patients with CNS depression early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin).
Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
Psychiatric Follow-up
Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
Pediatric Management
The principles of management of pediatric and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
Pharmacology
Mechanism of Action
Amitriptyline acts primarily as a serotonin-norepinephrine reuptake inhibitor, with strong actions on the serotonin transporter and moderate effects on the norepinephrine transporter.[1][2] It has negligible influence on the dopamine transporter and therefore does not affect dopamine reuptake, being nearly 1,000 times weaker on it than on serotonin.[2] It is metabolised to nortriptyline — a more potent and selective norepinephrine reuptake inhibitor — which may hence compliment its effects on norepinephrine reuptake.
Amitriptyline additionally functions as a 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, 5-HT7, α1-adrenergic, H1, H2,[3] H4,[4][5] and mACh receptorantagonist, and σ1 receptor agonist.[6][7][8][9] It has also been shown to be a relatively weak NMDA receptor negative allosteric modulator at the same binding site as phencyclidine.[10] Amitriptyline inhibits sodium channels, L-type calcium channels, and Kv1.1, Kv7.2, and Kv7.3 voltage-gated potassium channels, and therefore acts as a sodium, calcium, and potassium channel blocker as well.[11][12]
Recently, amitriptyline has been demonstrated to act as an agonist of the TrkA and TrkB receptors.[13] It promotes the heterodimerization of these proteins in the absence of NGF and has potent neurotrophic activity both in-vivo and in-vitro in mouse models.[13][14] These are the same receptors BDNF activates, an endogenous neurotrophin with powerful antidepressant effects, and as such this property may contribute significantly to its therapeutic efficacy against depression. Amitriptyline also acts as FIASMA (functional inhibitor of acid sphingomyelinase).[15]
Structure
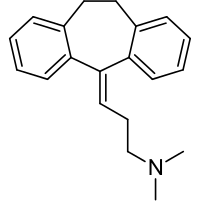
Amitriptyline HCl, a dibenzocycloheptadiene derivative, is a white, or practically white, odorless, crystalline compound which is freely soluble in water and alcohol.
It is designated chemically as 10,11-Dihydro-N,N-dimethyl-5H-dibenzo[a,d] cycloheptene-Δ5, γ-propylamine hydrochloride. It has the following structural formula: amitriptyline HCl chemical structure
Each tablet for oral administration contains 10, 25, 50, 75, 100, or 150 mg amitriptyline hydrochloride. Inactive ingredients include colloidal silicon dioxide, hydroxypropyl cellulose, hydroxypropyl methylcellulose, lactose (monohydrate), magnesium stearate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch (corn) and titanium dioxide. The 10 mg also includes D&C Red #27 Aluminum Lake, D&C Yellow #10 Aluminum Lake and FD&C Blue #1 Aluminum Lake; 25 mg – D&C Yellow #10 Aluminum Lake, FD&C Blue #1 Aluminum Lake and FD&C Red #40 Aluminum Lake; 50 mg – FD&C Blue #2 Aluminum Lake and FD&C Red #40 Aluminum Lake; 75 mg – D&C Red #7 Calcium Lake and FD&C Blue #2 Aluminum Lake; 100 mg – D&C Red #30 Aluminum Lake and D&C Yellow #10 Aluminum Lake; 150 mg – D&C Yellow #10 Aluminum Lake, FD&C Blue #1 Aluminum Lake and FD&C Red #40 Aluminum Lake.
Pharmacodynamics
Amitriptyline HCl is an antidepressant with sedative effects. Its mechanism of action in man is not known. It is not a monoamine oxidase inhibitor and it does not act primarily by stimulation of the central nervous system.
Amitriptyline inhibits the membrane pump mechanism responsible for uptake of norepinephrine and serotonin in adrenergic and serotonergic neurons. Pharmacologically, this action may potentiate or prolong neuronal activity since reuptake of these biogenic amines is important physiologically in terminating transmitting activity. This interference with reuptake of norepinephrine and/or serotonin is believed by some to underlie the antidepressant activity of amitriptyline.
Pharmacokinetics
Amitriptyline is readily absorbed from the gastrointestinal tract and is extensively metabolised on first-pass through the liver. It is metabolised mostly via CYP2D6, CYP3A4, CYP2C9-mediated N-demethylation into nortriptyline, which is another tricyclic antidepressant in its own right. It is 96% bound to plasma proteins, nortriptyline is 93-95% bound to plasma proteins.It is mostly excreted in the urine (around 30-50%) as metabolites either free or as glucuronide and sulfate conjugates. Small amounts are also excreted in faeces.
Nonclinical Toxicology
There is limited information regarding Amitriptyline Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Amitriptyline Clinical Studies in the drug label.
How Supplied
Amitriptyline hydrochloride tablets, USP for oral administration are available as:
10 mg: Round, film-coated pink tablets, debossed GG 40 on one side and plain on the reverse side, and supplied as:
NDC 0781-1486-31 bottles of 30
NDC 0781-1486-01 bottles of 100
NDC 0781-1486-10 bottles of 1000
25 mg: Round, film-coated light green tablets, debossed GG 44 on one side and plain on the reverse side, and supplied as:
NDC 0781-1487-31 bottles of 30
NDC 0781-1487-01 bottles of 100
NDC 0781-1487-10 bottles of 1000
50 mg: Round, film-coated brown tablets, debossed GG 431 on one side and plain on the reverse side, and supplied as:
NDC 0781-1488-31 bottles of 30
NDC 0781-1488-01 bottles of 100
NDC 0781-1488-10 bottles of 1000
75 mg: Round, film-coated purple tablets, debossed GG 451 on one side and plain on the reverse side, and supplied as:
NDC 0781-1489-31 bottles of 30
NDC 0781-1489-01 bottles of 100
NDC 0781-1489-10 bottles of 1000
100 mg: Round, film-coated orange tablets, debossed GG 461 on one side and plain on the reverse side, and supplied as:
NDC 0781-1490-31 bottles of 30
NDC 0781-1490-01 bottles of 100
NDC 0781-1490-10 bottles of 1000
150 mg: Capsule shaped, film-coated light green tablets, debossed GG 450 on one side and plain on the reverse side, and supplied as:
NDC 0781-1491-31 bottles of 30
NDC 0781-1491-01 bottles of 100
NDC 0781-1491-10 bottles of 1000
Storage
Store at 20º-25ºC (68º-77ºF) (see USP Controlled Room Temperature).
Dispense in a tight, light-resistant container.
Images
Drug Images
{{#ask: Page Name::Amitriptyline |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Amitriptyline |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with amitriptyline hydrochloride and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions” is available for amitriptyline hydrochloride. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking amitriptyline hydrochloride.
Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient’s prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
While on therapy with amitriptyline hydrochloride, patients should be advised as to the possible impairment of mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle.
Precautions with Alcohol
Alcohol-Amitriptyline interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Amitriptyline Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Amitriptyline Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Potency of antidepressants to block noradrenaline reuptake". CNS Forum. Retrieved 2013-02-16.
- ↑ 2.0 2.1 Tatsumi M, Groshan K, Blakely RD, Richelson E (December 1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". Eur. J. Pharmacol. 340 (2–3): 249–58. doi:10.1016/S0014-2999(97)01393-9. PMID 9537821.
- ↑ Albert Ellis; Gwynn Pennant Ellis (1 January 1987). Progress in Medicinal Chemistry. Elsevier. p. 56. ISBN 978-0-444-80876-9. Retrieved 27 November 2011.
- ↑ Nguyen T, Shapiro DA, George SR; et al. (March 2001). "Discovery of a novel member of the histamine receptor family". Molecular Pharmacology. 59 (3): 427–33. PMID 11179435.
- ↑ D. Sriram & P. Yogeeswari (1 September 2010). Medicinal Chemistry. Pearson Education India. p. 299. ISBN 978-81-317-3144-4. Retrieved 27 November 2011.
- ↑ Owens MJ, Morgan WN, Plott SJ, Nemeroff CB (December 1997). "Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites". J. Pharmacol. Exp. Ther. 283 (3): 1305–22. PMID 9400006.
- ↑ Alan F. Schatzberg, Charles B. (2006). Essentials of clinical psychopharmacology. American Psychiatric Pub. p. 7. ISBN 978-1-58562-243-6.
- ↑ Rauser L, Savage JE, Meltzer HY, Roth BL (October 2001). "Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxytryptamine(2C) receptor". J. Pharmacol. Exp. Ther. 299 (1): 83–9. PMID 11561066.
- ↑ Werling LL, Keller A, Frank JG, Nuwayhid SJ (October 2007). "A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder". Exp. Neurol. 207 (2): 248–57. doi:10.1016/j.expneurol.2007.06.013. PMID 17689532.
- ↑ Sills MA, Loo PS (July 1989). "Tricyclic antidepressants and dextromethorphan bind with higher affinity to the phencyclidine receptor in the absence of magnesium and L-glutamate". Mol. Pharmacol. 36 (1): 160–5. PMID 2568580.
- ↑ Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C (January 1998). "Inhibition of neuronal Na+ channels by antidepressant drugs". J. Pharmacol. Exp. Ther. 284 (1): 208–14. PMID 9435180.
- ↑ Punke MA, Friederich P (May 2007). "Amitriptyline is a potent blocker of human Kv1.1 and Kv7.2/7.3 channels". Anesthesia and Analgesia. 104 (5): 1256–1264. doi:10.1213/01.ane.0000260310.63117.a2. PMID 17456683.
- ↑ 13.0 13.1 Jang SW, Liu X, Chan CB, Weinshenker D, Hall RA, Xiao G, Ye K (June 2009). "Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity". Chem. Biol. 16 (6): 644–56. doi:10.1016/j.chembiol.2009.05.010. PMC 2844702. PMID 19549602.
- ↑ "Pharmaceutical Information - AMITRIPTYLINE". RxMed. Retrieved 2013-02-16.
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P (2011). Riezman, Howard, ed. "Identification of novel functional inhibitors of acid sphingomyelinase". PLoS ONE. 6 (8): e23852. doi:10.1371/journal.pone.0023852. PMC 3166082. PMID 21909365.
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_675440085.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=2102;V |Pill Dosage=25 mg |Pill Color=Yellow|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Aphena Pharma Solutions - Tennessee, Inc. |NDC=675440085
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Perphenazine_and_Amitriptyline_Hydrochloride_NDC_03780042.jpg |Drug Name=Perphenazine and Amitriptyline Hydrochloride |Pill Ingred=PERPHENAZINE[PERPHENAZINE];AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=MYLAN;727 |Pill Dosage=10 mg |Pill Color=Blue|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780042
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Perphenazine_and_Amitriptyline_Hydrochloride_NDC_03780073.jpg |Drug Name=Perphenazine and Amitriptyline Hydrochloride |Pill Ingred=PERPHENAZINE[PERPHENAZINE];AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=MYLAN;73 |Pill Dosage=50 mg |Pill Color=Purple|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780073
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_03782610.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M77 |Pill Dosage=10 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03782610
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Chlordiazepoxide_and_Amitriptyline_Hydrochloride_NDC_03780211.jpg |Drug Name=Chlordiazepoxide and Amitriptyline Hydrochloride |Pill Ingred=CHLORDIAZEPOXIDE[CHLORDIAZEPOXIDE];AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=MYLAN;211 |Pill Dosage=12.5 mg |Pill Color=Green|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780211
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Perphenazine_and_Amitriptyline_Hydrochloride_NDC_03780442.jpg |Drug Name=Perphenazine and Amitriptyline Hydrochloride |Pill Ingred=PERPHENAZINE[PERPHENAZINE];AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=MYLAN;442 |Pill Dosage=25 mg |Pill Color=Purple|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780442
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_03782685.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M;38 |Pill Dosage=100 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03782685
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_03782695.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M39 |Pill Dosage=150 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=17 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03782695
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_06032212.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=2101;V |Pill Dosage=10 mg |Pill Color=Blue|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032212
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_06032213.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=2102;V |Pill Dosage=25 mg |Pill Color=Yellow|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032213
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_06032217.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=2106;V |Pill Dosage=150 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=17 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032217
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_07811489.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=GG451 |Pill Dosage=75 mg |Pill Color=Purple|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811489
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_07811490.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=GG461 |Pill Dosage=100 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811490
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_09040202.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=GG;431 |Pill Dosage=50 mg |Pill Color=Brown|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09040202
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_510790107.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M;51 |Pill Dosage=25 mg |Pill Color=Green|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Institutional Inc. |NDC=510790107
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_510790131.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M77 |Pill Dosage=10 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Institutional Inc. |NDC=510790131
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_510790133.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M;36 |Pill Dosage=50 mg |Pill Color=Brown|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Institutional Inc. |NDC=510790133
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_510790147.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M;37 |Pill Dosage=75 mg |Pill Color=Blue|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Institutional Inc. |NDC=510790147
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_510790563.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M;38 |Pill Dosage=100 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Institutional Inc. |NDC=510790563
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_675440206.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=2101;V |Pill Dosage=10 mg |Pill Color=Blue|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Aphena Pharma Solutions - Tennessee, Inc. |NDC=675440206
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Chlordiazepoxide_and_Amitriptyline_Hydrochloride_NDC_03780277.jpg |Drug Name=Chlordiazepoxide and Amitriptyline Hydrochloride |Pill Ingred=CHLORDIAZEPOXIDE[CHLORDIAZEPOXIDE];AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=MYLAN;277 |Pill Dosage=25 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780277
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Perphenazine_and_Amitriptyline_Hydrochloride_NDC_03780330.jpg |Drug Name=Perphenazine and Amitriptyline Hydrochloride |Pill Ingred=PERPHENAZINE[PERPHENAZINE];AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=MYLAN;330 |Pill Dosage=10 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780330
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Perphenazine_and_Amitriptyline_Hydrochloride_NDC_03780574.jpg |Drug Name=Perphenazine and Amitriptyline Hydrochloride |Pill Ingred=PERPHENAZINE[PERPHENAZINE];AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=MYLAN;574 |Pill Dosage=25 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780574
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_06032214.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=2103;V |Pill Dosage=50 mg |Pill Color=Brown|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032214
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_06032215.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=2104;V |Pill Dosage=75 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032215
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_06032216.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=2105;V |Pill Dosage=100 mg |Pill Color=Red|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032216
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_07811486.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=GG40 |Pill Dosage=10 mg |Pill Color=Pink|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811486
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_07811487.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=GG44 |Pill Dosage=25 mg |Pill Color=Green|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811487
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_07811488.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=GG431 |Pill Dosage=50 mg |Pill Color=Brown|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811488
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_675440253.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=AMITRIPTYLINE HYDROCHLORIDE[AMITRIPTYLINE]|+sep=; |Pill Imprint=2103;V |Pill Dosage=50 mg |Pill Color=Brown|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Aphena Pharma Solutions - Tennessee, Inc. |NDC=675440253
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_03782625.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M;51 |Pill Dosage=25 mg |Pill Color=Green|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03782625
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_03782650.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M;36 |Pill Dosage=50 mg |Pill Color=Brown|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03782650
}}
{{#subobject:
|Page Name=Amitriptyline |Pill Name=Amitriptyline_Hydrochloride_NDC_03782675.jpg |Drug Name=Amitriptyline Hydrochloride |Pill Ingred=Amitriptyline Hydrochloride[Amitriptyline]|+sep=; |Pill Imprint=M;37 |Pill Dosage=75 mg |Pill Color=Blue|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03782675
}}
{{#subobject:
|Label Page=Amitriptyline |Label Name=Atca1.png
}}
{{#subobject:
|Label Page=Amitriptyline |Label Name=Atca6.png
}}
{{#subobject:
|Label Page=Amitriptyline |Label Name=Atca5.png
}}
{{#subobject:
|Label Page=Amitriptyline |Label Name=Atca4.png
}}
{{#subobject:
|Label Page=Amitriptyline |Label Name=Atca3.png
}}
{{#subobject:
|Label Page=Amitriptyline |Label Name=Atca2.png
}}
{{#subobject:
|Label Page=Amitriptyline |Label Name=Amip.jpeg
}}