Ventricular septal defect: Difference between revisions
Redmond111 (talk | contribs) |
No edit summary |
||
| Line 16: | Line 16: | ||
}} | }} | ||
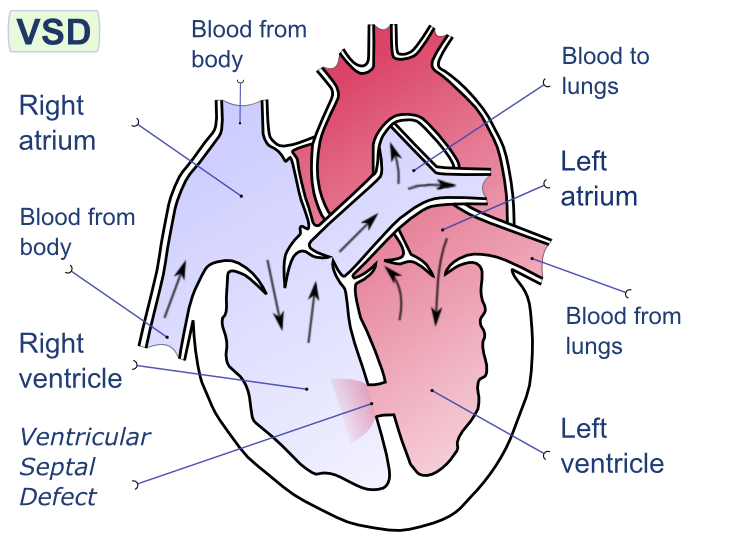
[[Image:Ventricular septal defect-en.png|thumb|200px|Ventricular septal defect]] | [[Image:Ventricular septal defect-en.png|thumb|200px|Ventricular septal defect]] | ||
{{SI}} | {{SI}} | ||
{{CMG}} and Leida Perez, M.D. | {{CMG}} and Leida Perez, M.D. | ||
Revision as of 13:15, 1 February 2011
| Ventricular septal defect | ||
 | ||
|---|---|---|
| Echocardiographic image of a moderate ventricular septal defect in the mid-muscular part of the septum. The trace in the lower left shows the flow during one complete cardiac cycle and the red mark the time in the cardiac cycle that the image was captured. Colours are used to represent the velocity of the blood. Flow is from the left ventricle (right on image) to the right ventricle (left on image). The size and position is typical for a VSD in the newborn period. | ||
| ICD-10 | Q21.0 | |
| ICD-9 | 745.4 | |
| DiseasesDB | 13808 | |
| eMedicine | med/3517 | |
| MeSH | C14.240.400.560.540 | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] and Leida Perez, M.D.
Associate Editor-in-Chief: Keri Shafer, M.D. [2]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
A ventricular septal defect (or VSD) is a defect in the ventricular septum (the wall dividing the left and right ventricles of the heart). The ventricular septum consists of a muscular (inferior) and membranous portion (superior). The membranous portion (which is close to the atrioventricular node) is most commonly affected.[1]
Congential VSDs are collectively the most common congenital heart defect.[2]
Epidemiology and Demographics
The ventricular septal defect is the most common congenital cardiac malformation with an incidence of 1.5 to 2.5 per 1,000 live births, corresponding to 30% of all newborns with a congenital heart defect. There is no predilection based on sex.
The several known risk factors for VSD, including a family history of congenital heart disease and exposure to certain drugs, infectious agents, and maternal metabolic disturbances, explain few cases. Incidence rates are similar in different races and seasons and are unrelated to maternal age, birth order, sex, and socioeconomic status. Congential VSDs are frequently associated with other congential conditions, such as Down syndrome. [3]
Only in the United States, there are approximately 1 million adults with congenital heart disease, with 20,000 new patients reaching adolescence each year. Due to the improvement in early diagnosis in childhood and improved medical, surgical and ICU care, the number of adults will continue to rise. However, despite improved survival to adulthood, many patients will continue to have problems with residual shunts, valvular heart disease, ventricular dysfunction, heart failure and arrhythmias. The risk of sudden death in adults with congenital heart disease is nearly 25-50 times greater than would be expected for their age.
In adults, a VSD can form a few days after a myocardial infarction (heart attack) due to mechanical tearing of the septal wall, before scar tissue forms, when macrophages start remodeling the dead (heart) tissue.
Anatomy
Normal Ventricular Septum Anatomy
In the normal heart, the ventricular septum (VS) is mostly muscular. The fibrous component is the so-called membranous septum, and is only a small part of the VS. It is integrated into the aortic root, and is made of fibrous tissue. It is located inferiorly to the inter-leaflet triangle that fills the space between the ascending hinges of the non-coronary and right coronary leaflets of the aortic valve [4]
When transilluminated from the right atrioventricular aspect, it can be seen, in most cases, to be crossed by the hinge of the tricuspid valve, thus dividing it into atrioventricular and interventricular components. It is then the position of the hinge which determines the proportions which are atrioventricular as opposed to interventricular [5]
The atrioventricular component of the septum is itself pierced by the atrioventricular conduction axis as it passes from the apex of the triangle of Koch to reach the crest of the muscular septum
Locations of VSD
There are two basic different locations of VSD to be distinguished:
a. VSD located over the Crista Supraventricularis (less frequent, 4 -5 % of all VSD), are also called SUPRACRISTAL VSD or subaortic. Defect located in the outflow tract of the right ventricle.
Both the right and posterior coronary aortic valves are directly over the defect. Many times there is aortic insufficiency and rarely the aortic orifice can override the septal defect.
b. VSD located below the Crista Supraventricularis (more frequent). Also known as infracristal or subpulmonar. The defects located in front of the Conus Papillary muscle and behind the Crista are the most common variety.
There are defects behind the Conus Papilary muscle which are the posterior and basal VSD. Usually in this variety the defect size is larger than in the previous type.
There is a third variety located in the muscular interventricular septum (single or multiple orifices) (Roger disease). When the muscular VSD is multiple, resemble Swiss cheese.
Muscular VSD are bounded by muscles and not immediately related to the valves. They can be located in the apical, central, or outlet region of the ventricular septum.
Pathophysiology
In this lesion, a persistent opening in the upper interventricular septum resulting from failure of fusion with the aortic septum allows blood to flow from the high pressure left ventricle into the low pressure chamber or right ventricle. The subsequent natural history and pathophysiology depend on the size of the defect and the magnitude of left-to-right shunting. Small defects (QP/QS less than 1.5) maybe asymptomatic, but with the high risk for bacterial endocarditis. Large defects are associated with left ventricular failure. Chronic but more moderate left-to-right shunts may lead to pulmonary vascular disease and right sided failure.
The primary variable is the size of the defect. As a child grows, the relative size of the defect may decrease and the defect may even close spontaneously in early childhood.
During the first few months of life the PVR decreases, and the magnitude of left-to-right shunt increases. After the first few months the degree of shunting is dependent on the size of the defect.
Presentations in the adult or adolescent: a) small defect without significant left-to-right shunting
b) a large defect with severe pulmonary hypertension and cyanosis due to right-to-left shunt.
c) a large defect with a large left-to-right shunt that has induced secondary infundibular stenosis (tough to differentiate from tetralogy of Fallot).
Small VSDs
A high resistance to flow across the VSD due to the large pressure difference between the two ventricles. There is a small left-to-right shunt (Qp/Qs < 1.5) and a normal ratio of PA to systemic pressures.
There is little or no increase in the pulmonary vascular resistance. A holosystolic murmur is present due to the pressure gradient across the defect. The majority of these defects close during the first three years of life.
Medium-Sized VSDs
There is a moderate shunt left-to-right present(Qp/Qs = 1.5-2.0) that still has some resistance to flow across the defect. There is also volume overload of the LA and the LV and LVH. There may therefore be a middiastolic mitral murmur and a third heart sound (S3). The ratio of the PA systolic pressure to the systemic pressure is <.5.
The area of the defect is usually less than 1 cm2/m2 of body surface area and is unusual for this group to have a marked increase in PVR. In some cases and depending on the type of VSD, as the child becomes older, the relative size of the defect will decrease.
Large VSDs
There is a large defect on the ventricular septum, > 1 cm2/m2 of BSA, with a large shunt left-to-right (Qp/Qs is > 2), causing volume overload of the LV, which may result in its failure. The defect may approximate the size of the aortic orifice.
The ratio of the PA pressure to the systemic pressure is >.5. Produce the same clinical findings as moderate sized VSD but also pulmonary hypertension.
Rarely spontaneously close, and these patients either die, or progress to adolescence or adulthood with severe pulmonary hypertension or with secondary protective infundibular pulmonary stenosis.
In the group with severe pulmonary hypertension, the left-to-right shunt decreases and the degree of right-to-left shunting increases with accompanying cyanosis (i.e. they develop Eisenmenger's syndrome).
Protective infundibular stenosis may also result in reversal of the shunt, and may be indistinguishable clinically from tetralogy of Fallot.
Genetics
The frequent association between arch abnormalities and significant conal VSDs suggests a common mechanism involving a chromosome band 22q11 microdeletion. Deletions in this area have not been linked with isolated supracristal VSDs.
Clinical Features
Depends on the size of the defect and the pulmonary vascular resistence (PVR). Defects in the muscular septum and subtricuspid defects frequently close or get smaller with time. Subaortic defects do not close spontaneously because the superior border is the aortic valve.
Clinical Features of Small VSDs
Generally, the course is benign throughout infancy and childhood.
Clinical Features of Medium-Sized VSDs
Common in infancy, rarely seen in adults.
More common in adulthood is a medium-sized left to right shunt of other causes, either a large VSD with protective infundibular stenosis, or a large VSD partially occluded by a septal leaflet of the tricuspid valve.
Clinical Features of Large VSDs
From age 1 to 12 months severe symptoms are due to LV failure secondary to a large left-to-right shunt.
a) tachypnea
b) excess sweating
c) fatigue with feeding
d) all these become progressively worse
e) respond well to medical therapy and the development of LVH also allows the LV to handle larger flows.
From 6 to 24 months, there are decreased symptoms due to a decrease in left-to-right shunting. Causes of the decreased shunt include:
a) may be due to spontaneous closure of the defect
b) there may be a progressive increase in the PVR (the most frequent cause).
c) occasionally there is protective hypertrophy of the outflow tract of the RV. This increases resistance through the pulmonary circuit and decreases the shunt to that of a moderate-sized defect. Usually this obstruction becomes severe and the patient develops a tetralogy of Fallot type of syndrome.
These patients must be studied during the first year of life. It is within the first year that these patients develop pulmonary vascular obstructive disease, and unless surgical repair is undertaken they become inoperable.
Because of the rise in the PVR, the majority of these patients become fairly asymptomatic at age 12 to 24 months, but in adolescence, the pulmonary vascular resistance becomes so high that right-to-left shunting develops and the patients develop cyanosis and the "Eisenmenger's complex".
The definition of Eisenmenger's syndrome is officially any defect which allows free communication between the pulmonary and systemic circuits with a predominant right-to-left shunt secondary to a large rise in the PVR. Features include:
a) chest pain resembling angina
b) exertional syncope
c) hemoptysis
d) cerebral thrombosis related to the high hematocrit value
e) cerebral abscesses related to paradoxic emboli
f) death in the third decade, usually is sudden, particularly high mortality rate in pregnant women.
Physical Examination
Small VSD:
A systolic thrill may be palpable along the left sternal border and a loud holosystolic murmur (harsher quality than that of MR)localized to the left lower sternal border. In patients with small muscular defects, the murmur may end in mid systole because of systolic contraction of the septal musculature.
Medium-Sized VSD:
The rare patient who presents with a medium-sized defect or a moderate left-to-right shunt also may have a third heart sound (S3) and a short early middiastolic rumble due to increased left-sided volume overload.
Large-Sized VSD with Pulmonary Obstructive Disease:
In the first 2 years of age the patients have signs of left sided volume overload. After age 2 old, the patients have signs and symptoms of progressive pulmonary vascular obstructive disease. As a consequence, poor growth and left anterior thorax may bulge outward early. JVD may be elevated due to RV failure.
In the first two years there is a prominent LV impulse, but with the development of pulmonary hypertension, this LV prominence is diminished and cyanosis is present, worsens with effort and with time.
Electrocardiogram
Small VSD: EKG is normal. A few patients will have an rsr' in V1.
Medium-sized VSD: in the adolescence with this type of defect, or a moderate left-to-right shunt, the EKG may show LVH, and in addition there may be mild RVH in lead V1 with an rsR' pattern.
Large VSD: In adults or adolescence with a large VSD and pulmonary vascular obstructive disease, LVH is absent because volume overload of the LV is no longer present. At this point there is either an rsR' pattern in the right precordial leads, or more commonly, a tall monophasic R wave in the right precordial leads reflecting RVH. Also deep S waves in the lateral precordial leads and tall peaked P waves.
In patients with an acquired infundibular stenosis, the EKG shows a pattern of RVH similar to the tracing of patients with tetralogy of Fallot.
Chest X Ray
Small VSD:
Normal heart size and pulmonary vascularity.
Medium-sized defect (or moderate left-to-right shunt):
a) slight cardiomegaly
b) increased pulmonary vascular markings in both the central and the peripheral portions of the lung field.
Large VSDs:
a) LVH has regressed in adults, apex is tilted upward reflecting RVH.
b) dilated proximal pulmonary vessels, pruned peripheral vessels
Echocardiogram
Two dimensional echo(2D) can be used to visualize the defects, and with pulsed wave and color doppler the sensitivity is 90%. The velocity across the defect can be used to calculate the gradient, and calculate the RV and PA pressures.
<youtube v=1u2EK9S8q5s />
Ventricular Septal Defect After Myocardial Infarction 1
<googlevideo>6643787626215386202&hl=en</googlevideo>
Ventricular Septal Defect After Myocardial Infarction 2
<googlevideo>-5841159884627614577&hl=en</googlevideo>
For more info and images, see Echo in Ventricular Septal Defect
Cardiac Catheterization
Criteria for Cardiac Catheterization:
1. The Mayo Clinic recommends cardiac catheterization for all adults and adolescents to quantify the degree of pulmonary vascular obstruction, except in those who clearly appear to have a small VSD.
2. Infants suspected of having a large defect should undergo cardiac catheterization in the first year of life which should allow surgical correction prior to the onset of permanent pulmonary vascular obstructive disease.
Treatment
Management of the Infant
Small VSD
In infants with a small defect, reassurance of parents and close follow-up. Antibiotic use as prophylaxis for bacterial endocarditis.
Moderate and Large VSD
There is a greater risk of operative closure in early infancy than at age 1 to 2 years.
The diagnosis in an infant is usually made in the first few months and cardiac catheterization is performed:
a) if the defect is sufficiently large to allow equalization of pressures, (PAP/SP > .75) then an operation is urgently needed whenever cardiac failure occurs and is not responsive to medical management.
b) the risk of death from closing the defect in this circumstance is 10% to 20%, but this is less than the risk of leaving the defect unrepaired.
c) if CHF can be controlled medically, then careful observation in warranted with repeat cardiac catheterization at 12 to 15 months.
d) If the PAP/SP is > .75, then surgery is indicated, because a delay in closure may lead to progressive pulmonary vascular obstructive disease.
e) Further delay will not decrease the risk below that at 18 to 24 months (2% operative mortality).
f) The likelihood of closure of the defect after this age is remote.
g) If repeat cardiac catheterization at 12 to 15 months suggests that the defect is becoming smaller, (PAP/SP < .75), then further postponement of the operation is advisable. The likelihood of development of pulmonary obstructive disease is remote, the defect is also likely to continue to diminish in size.
h) Similarly, if at the original cardiac catheterization the PAP/SP was < .75, then postponement would be advisable.
i) Patients with PAP/SP < .75 should be followed until age 4 when they should undergo repeat cardiac catheterization.
j) At that time, even if the PAP/SP < .5, then operative closure of the VSD is advised if a large amount of pulmonary blood flow is present (Qp/Qs = 1.5 to 2.0).
k) this is because the hemodynamic burden is significant and may handicap the growing child or cause irreversible LV changes and because the defect is of moderate size and is not showing evidence of spontaneous closure.
l) If Qp/Qs is 1.3 to 1.5, then further observation is warranted because of the hope that closure could still occur.
m) if Qp/Qs is < 1.3, then closure could be avoided altogether
Management of the Adolescent and Adult Patient
In general there are three presentations:
a) Small VSD with a PAP/SP that is normal, a Qp/Qs < 1.3. In this cases operation is unnecessary.
b) If Qp/Qs 1.3 to 1.5 the indications for surgery are borderline.
Moderate-sized VSD or moderate left-to-right shunt with PAP/SP < 0.5 and Qp/Qs 1.5 to 2.0, then operation is advised.
c) Large VSDs with PAP/SP > 0.75 but with small Qp/Qs due to significant pulmonary vascular obstructive disease. Deemed inoperable when the resistance Rp, is greater than 10 unitsx m2. The pulmonary hypertension may persist postoperatively.
Lung biopsy does not add information that is helpful in making this decision.
The management of the patient with acquired infundibular stenosis is the same as for the patient with tetralogy of Fallot.
Surgical technique for Repair of Perimembranous VSD
a) Perimembranous VSD is repaired on cardiopulmonary bypass with ischemic arrest. Device closure is rarely used in the United States because of the reported incidence of early and late onset complete heart block after device closure, presumably secondary to device trauma to the AV node. b) Surgical exposure is achieved through the right atrium. The tricuspid valve septal leaflet is retracted or incised to expose the defect margins. c) Several patch materials are available, including native pericardium, bovine pericardium, PTFE (Goretex(tm) or Impra(tm), or dacron. d) Suture techniques include horizontal pledgeted mattress sutures, and running polypropylene suture. e) Critical attention is necessary to avoid injury to the conduction system located on the left ventricular side of the interventricular septum near the papillary muscle of the conus. f) Care is taken to avoid injury to the aortic valve with sutures. g) The heart is extensively deaired by venting blood through the aortic cardioplegia site, and by infusing Carbon Dioxide into the operative field to displace air. h) Intraoperative transesophageal echocardiography is used to confirm secure closure of the VSD, function of the aortic valve, ventricular function, and the elimination of all air from the left side of the heart. i) The sternum is closed, with potential placement of a local anesthetic infusion catheter under the fascia, to stabilize postoperative pain control. j) A video of Perimembranous VSD repair, including the operative technique, and the daily postoperative recovery, can be seen here: VSD Repair, Perimembranous Ventricular Septal Defect Repair Video
Post-operative Treatment
Post-operative course:
The operative mortality for an elective repair is less than 2%. It is increased by the presence of associated anomalies, multiple defects, or if there is severe pulmonary hypertension.
Late follow-up shows that their life expectancy is restored to that of age matched controls (except in those over the age of three with severe pulmonary hypertension).
There is a residual defect in 14% to 25% of patients which is hemodynamically insignificant, and a persistent RBBB in the majority of patients due to disruption of the Purkinje fibers.
In patients over 3 at the time of the operation, there is often residual and progressive pulmonary hypertension and or residual ventricular dysfunction.
The risk of endocarditis following closure is similar to that in the general population. Because small defects are frequent, antibiotic prophylaxis is still recommended.
References
- ↑ Anderson RH, Ho SY, Becker AE. Anatomy of the human atrioventricular junctions revisited. Anatomical Record 2000;260:81-91
- ↑ Allwork SP, Anderson RH. Developmental anatomy of the membranous part of the ventricular septum in the human heart. Br Heart J 1979; 41:275-280
- ↑ Giuliani et al, Cardiology: Fundamentals and Practice, Second Edition, Mosby Year Book, Boston, 1991.
- ↑ Anderson RH, Ho SY, Becker AE. Anatomy of the human atrioventricular junctions revisited. Anatomical Record 2000;260:81-91
- ↑ Allwork SP, Anderson RH. Developmental anatomy of the membranous part of the ventricular septum in the human heart. Br Heart J 1979; 41:275-280
External Links for VSD
- Pediatric Heart Surgery
- The Congenital Heart Surgery Video Project
- VSD Repair, Perimembranous Ventricular Septal Defect
- VSD Repair Powerpoint™ Presentation
Acknowledgements and Initial Contributors to Page
Leida Perez, M.D. Redmond Burke M.D.