Ventricular assist device
Editor-In-Chief: Marv Slepian, M.D.; University of Arizona [1]; Juan A. Sanchez MD MPA [2], Chairman, The Stanley J. Dudrick Department of Surgery, Saint Mary's Hospital, Waterbury, CT
Associate Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3] Phone:617-632-7753
Overview
A Ventricular assist device, or VAD, is a type of artificial heart or assisted circulation[1] that is a mechanical device that is used to partially or completely replace the function of a failing heart. Some VADs are intended for short term use, typically for patients recovering from heart attacks or heart surgery, while others are intended for long term use (months to years and in some cases for life), typically for patients suffering from congestive heart failure.
VADs need to be clearly distinguished from artificial hearts, which are designed to completely take over cardiac function and generally require the removal of the patient's heart.
VADs are designed to assist either the right (RVAD) or left (LVAD) ventricle, or both at once (BiVAD). The choice of device depends on the underlying heart disease and the pulmonary arterial resistance which determines the load on the right ventricle. LVADs are most commonly used but when pulmonary arterial resistance is high, right ventricular assist becomes necessary. Long term VADs are normally used to keep patients alive with a good quality of life while they wait for a heart transplant (bridge to transplant). However, LVADs are sometimes used as destination therapy sometimes as a bridge to recovery.
Clinical practice guidelines suggest roles[2].
VADs can be:
- Percutaneous or transcutaneous. Examples include[2]
- Axial flow pumps, such as Impella
- Left atrial to femoral artery bypass pumps, such as the TandemHeart
- Implantable that allows a patient to be fully mobile. Examples include:
- HeartMate II which is a continuous-flow system with mechanical bearings
- HeartMate III which is a magnetically levitated device that is a pulsatile-flow system without mechanical bearings
Design
Overview
Most VADS operate on similar principles. A cannula is inserted into the apex of the appropriate ventricle. Blood passes through this to a pump and thence through a tube to the aorta in the case of an LVAD or to the pulmonary artery in the case of an RVAD. The pump is powered through a lead which connects it to a controller and power supply. In some cases there is also a tube to vent the pump to the outside air. The Jarvik 2000 operates slightly differently - the pump is actually located inside the left ventricle, its outflow passes through the apex of the ventricle to a tube which leads to the aorta.
Major distinguishing features between the different VADS are the pump (which can vary substantially in method of operation, size and placement), the controller, the materials used both for the pump and the associated tubes and cannulas and the lead between the pump and the controller/power supply.
Pumps
Pumps used in VADS can be divided into two main categories - pulsatile pumps, which as the name suggests mimic the natural pulsing action of the heart, and continuous flow pumps[3][4].
- First-generation devices are pulsatile volume displacement pumps that use positive displacement pumps. In some of these pumps the volume occupied by blood varies during the pumping cycle, and if the pump is contained inside the body then a vent tube to the outside air is required. Examples are Berlin Heart EXCOR, Novacor, LionHeart, HeartMate I
- Second-generation devices are axial, continuous flow pumps. Continuous flow VADs normally use either centrifugal pumps or axial flow pump. Both types have a central rotor containing permanent magnets. Controlled electric currents running through coils contained in the pump housing apply forces to the magnets which cause the rotors to rotate. In the centrifugal pumps the rotors are shaped to accelerate the blood circumferentially and thus cause it to move towards the outer rim of the pump whereas in the axial flow pumps the rotors are more or less cylindrical with blades that are more or less helical, causing the blood to be accelerated in the direction of the rotor's axis. Examples include MicroMed DeBakey, HeartMate II, Jarvik 2000, TandemHeart
- Third-generation devices are bearingless pumps. An important issue with continuous flow pumps is the method used to suspend the rotor. Early versions used solid bearings however newer pumps, some of which are approved for use in the EU use either electromagnetic or hydrodynamic suspension. Manufacturers claim that these methods of suspension not only virtually eliminate wear but also reduce damage to blood cells. Third-generation devices are bearingless pumps. Examples include CentriMag, DuraHeart, HeartMate III, Impella
Effectiveness
Clinical practice guidelines summarize indications[5].
Systematic reviews have assessed effectiveness[3][6] including comparison of effectiveness to intra-aortic balloon pump[7].
Among studies of artificial hearts, most original studies are not randomized; however, one randomized controlled trial found a trend, although insignificant, for benefit from the Impella ventricular assist device compare to intra-aortic balloon pumping[8].
Complications
Because the devices generally result in blood flowing over a non biologic surface, predisposing the blood to clotting, there is need for anticoagulation. There is one device, the Heartmate, which provides a biologic surface derived from fibrin and does not require long term anticoagulation; unfortunately, this biologic surface may predispose to infection.
VAD-related infection can be caused by a large number of different organisms:[9]
- Gram positive bacteria (Staphylococci especially Staph. aureus, Enterococci)
- Gram negative bacteria (Pseudomonas aeruginosa, Enterobacter species, Klebsiella species)
- Fungi especially Candida sp.
Treatment of VAD-related infection is exceedingly difficult and many patients die of infection despite optimal treatment. Initial treatment should be with broad spectrum antibiotics, but every effort must be made to obtain appropriate samples for culture. A final decision regarding antibiotic therapy must be based on the results of microbiogical cultures.
Other problems include immunosuppression, clotting with resultant stroke, and bleeding secondary to anticoagulation. It is interesting to note that some of the polyurethane components used in the devices cause the deletion of a subset of immune cells when blood comes in contact with them. This predisposes the patient to fungal and some viral infections necessitating appropriate prophylactic therapy.
History
The early VADs emulated the heart by using a "pulsatile" action where blood is alternately sucked into the pump from the left ventricle then forced out into the aorta. Devices of this kind include the Heartmate, which was approved for use in the US by the FDA in October 1994. These devices are commonly referred to as first generation VADs
More recent work has concentrated on continuous flow pumps, which can be roughly categorized as either centrifugal pumps or axial flow impeller driven pumps. These pumps have the advantage of greater simplicity resulting in smaller size and greater reliability. These devices are referred to as second generation VADs. A side effect is that their users need to carry documentation saying that the lack of a pulse does not mean that they are dead.
Third generation VADs suspend the impeller in the pump using either hydrodynamic or electromagnetic suspension, thus removing the need for bearings and reducing the number of moving parts to one.
Another technology undergoing clinical trials is the use of trans cutaneous induction to power and control the device rather than using percutaneous cables. Apart from the obvious cosmetic advantage this reduces the risk of infection and the consequent need to take preventative action. A pulsatile pump using this technology has CE Mark approval and is in clinical trials for US FDA approval.
A very different approach in the early stages of development is the use of an inflatable cuff around the aorta. Inflating the cuff contracts the aorta and deflating the cuff allows the aorta to expand - in effect the aorta becomes a second left ventricle. A proposed refinement is to use the patient's skeletal muscle, driven by a pacemaker, to power this device which would make it truly self contained. In any case it has substantial potential advantages in avoiding the need to operate on the heart itself and in avoiding any contact between blood and the device. Interestingly this approach involves a return to a pulsatile flow.
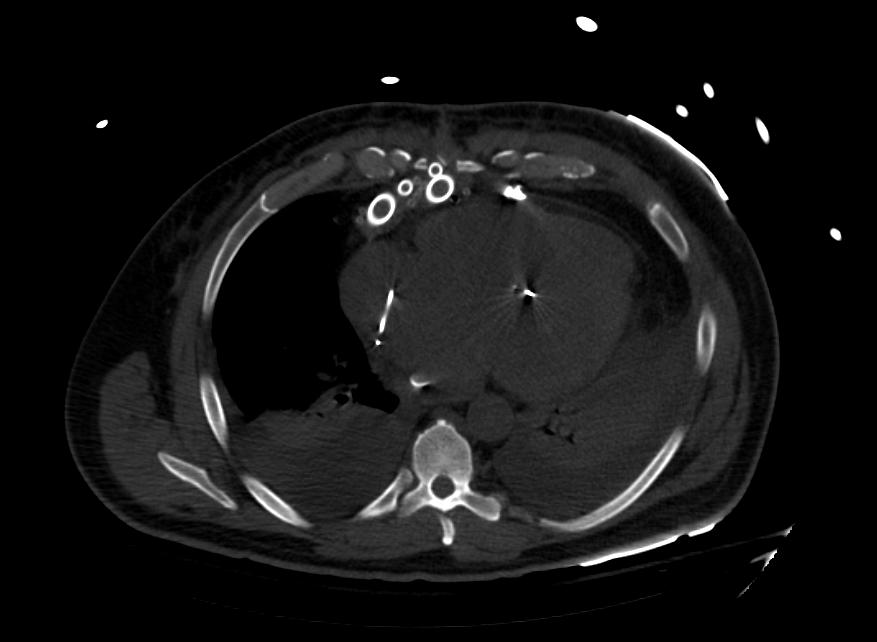
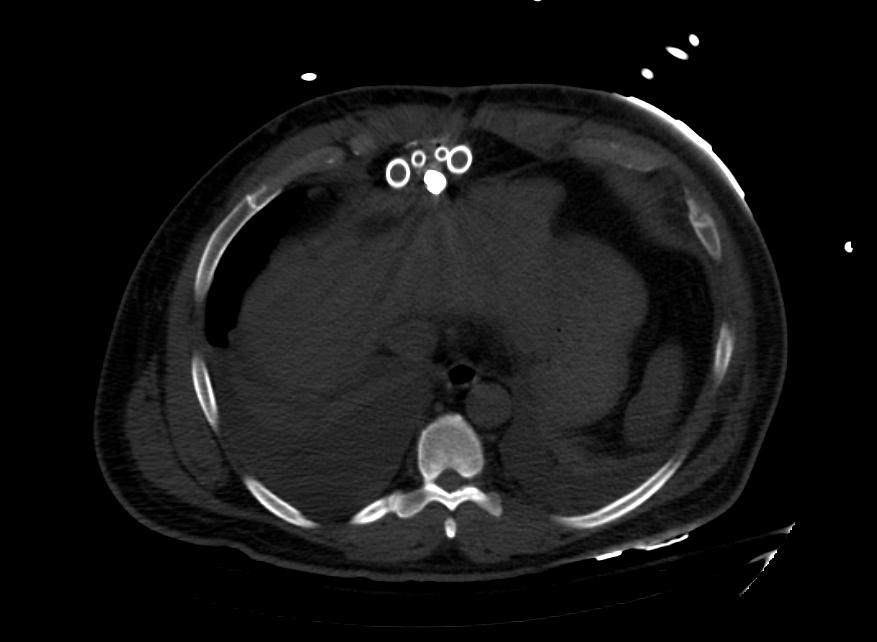
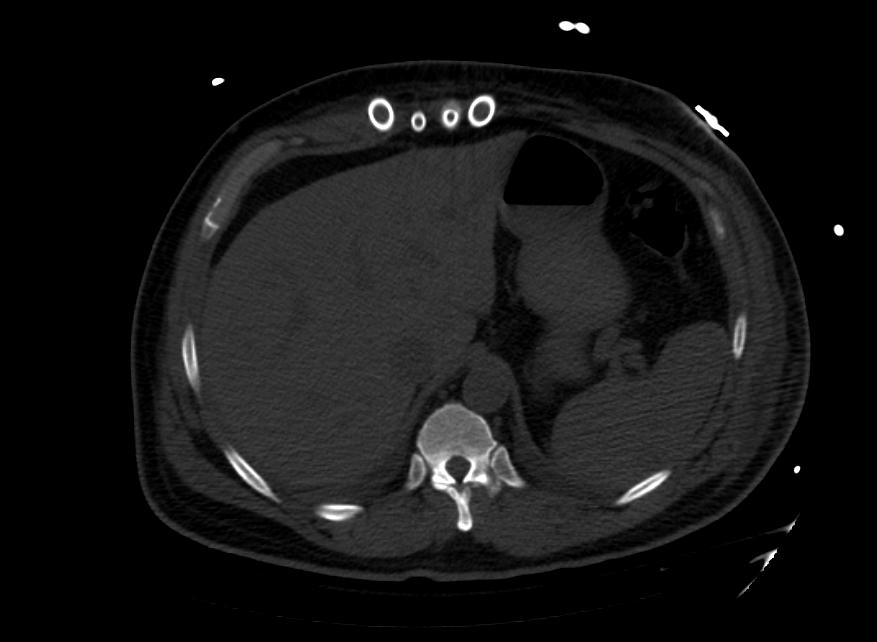
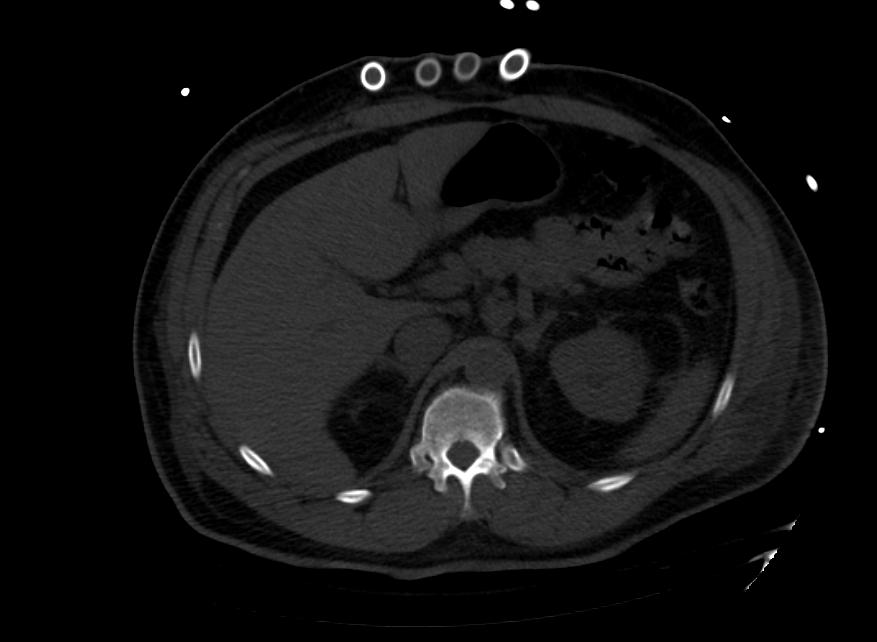
Images: Ventricular Assist Devices
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
List of implantable VAD devices
This is a partial list and may never be complete
Referenced additions are welcome
| Device | Manufacturer | Type | Approval Status as at December 2007 |
|---|---|---|---|
| Novacor | World Heart | Pulsatile | Approved for use in North America, European Union and Japan |
| Heartmate | Thoratec | Pulsatile | Approved for use in North America |
| Heartmate II | Thoratec | Rotor driven continuous axial flow, ball and cup bearings. | Approved for use in European Union. FDA approval for bridge to transplant achieved November 2007. |
| Incor | Berlin Heart | Continuous flow driven by a magnetically suspended axial flow rotor. | Approved for use in European Union. |
| Jarvik 2000 | Jarvik Heart | Continuous flow, axial rotor supported by ceramic bearings | Approved for use in the European Union. Clinical trials for FDA approval are planned. |
| MicroMed DeBakey VAD | MicroMed | Continuous flow driven by axial rotor supported by ceramic bearings | Approved for use in the European Union. The child version is approved for use in children in USA. Undergoing clinical trials in USA for FDA approval. |
| VentrAssist | Ventracor | Continuous flow driven by a hydrodynamically suspended centrifugal rotor. | Approved for use in European Union and Australia. Undergoing clinical trials for FDA approval |
| MTIHeartLVAD | MiTiHeart Corporation | Continuous flow driven by a magnetically suspended centrifugal rotor. | Yet to start clinical trials. |
| C-Pulse | Sunshine Heart | Pulsatile, driven by an inflatable cuff around the aorta | Yet to start clinical trials |
| HVAD | HeartWare | Similar to Ventracor - magnetic impellor similar to BLDCmotor | undergoing clinical trials for CE, applied to start FDA US trials
|
References
- ↑ Anonymous (2024), Assisted circulation (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ 2.0 2.1 Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK; et al. (2015). "2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention". J Am Coll Cardiol. 65 (19): e7–e26. doi:10.1016/j.jacc.2015.03.036. PMID 25861963.
- ↑ 3.0 3.1 Sutcliffe P, Connock M, Pulikottil-Jacob R, Kandala NB, Suri G, Gurung T; et al. (2013). "Clinical effectiveness and cost-effectiveness of second- and third-generation left ventricular assist devices as either bridge to transplant or alternative to transplant for adults eligible for heart transplantation: systematic review and cost-effectiveness model". Health Technol Assess. 17 (53): 1–499, v–vi. doi:10.3310/hta17530. PMC 4781283. PMID 24280231.
- ↑ https://www.openanesthesia.org/ventricular_assist_devices/
- ↑ Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK; et al. (2015). "2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care (Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie D'intervention)". Catheter Cardiovasc Interv. 85 (7): E175–96. doi:10.1002/ccd.25720. PMID 25851050.
- ↑ Briasoulis A, Telila T, Palla M, Mercado N, Kondur A, Grines C; et al. (2016). "Meta-Analysis of Usefulness of Percutaneous Left Ventricular Assist Devices for High-Risk Percutaneous Coronary Interventions". Am J Cardiol. 118 (3): 369–75. doi:10.1016/j.amjcard.2016.05.003. PMID 27265673.
- ↑ Shi W, Wang W, Wang K, Huang W (2019). "Percutaneous mechanical circulatory support devices in high-risk patients undergoing percutaneous coronary intervention: A meta-analysis of randomized trials". Medicine (Baltimore). 98 (37): e17107. doi:10.1097/MD.0000000000017107. PMC 6750338 Check
|pmc=value (help). PMID 31517843. - ↑ O'Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J; et al. (2012). "A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study". Circulation. 126 (14): 1717–27. doi:10.1161/CIRCULATIONAHA.112.098194. PMID 22935569.
- ↑ Gordon RJ, Quagliarello B, Lowy FD (2006). "Ventricular assist device-related infections". Lancet Infect Dis. 6 (7): 426&ndash, 37.
External links
- Nader Moazami, Patrick M. McCarthy Temporary Circulatory Support
- Eugene L. Kukuy, Mehmet C. Oz, Yoshifumi Naka Long-Term Mechanical Circulatory Support - a review of the subject as at 2003.
- Health Center Online VAD
- Mayo Clinic VAD
- FDA VAD
- Life without a pulse — news story about Canadian man with VAD
- Heart Pump Design Could Give Patients New Hope — A new counter-flow heart pump developed by Queensland University of Technology
- Heart pump improves quality of life in congestive heart failure patientsA rapid review of the medical literature and specialist opinion as at December 2005
- NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE (UK) Interventional procedures overview - short-term circulatory support with left ventricular assist devices as a bridge to cardiac transplantation or recovery A rapid review of the medical literature and specialist opinion as at December 2005
- Courtney J. Gemmato,Matthew D. Forrester,Timothy J. Myers,O.H. Frazier,Denton A. Cooley Thirty-Five Years of Mechanical Circulatory Support at the Texas Heart InstituteTex Heart Inst J 2005;32:168-77